Article contents
The effect of POSS-based block copolymer as compatibilizer on POSS/epoxy composites
Published online by Cambridge University Press: 28 January 2015
Abstract
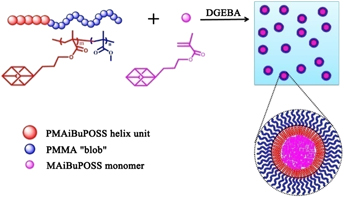
In this study, a novel hybrid block copolymer containing POSS (BCP), poly(methacrylisobutyl-POSS)-b-poly(methylmethacrylate) (PMAiBuPOSS-b-PMMA) was synthesized via reversible addition-fragmentation chain transfer (RAFT) polymerization. The structure and molecular weight were characterized via 1H NMR and GPC. BCP was creatively used as the compatibilizer to overcome the bad compatibility of epoxy and POSS in their blend system. SEM and dynamic mechanical thermal analyses (DMTA) were used to observe the surface morphology and thermal–mechanical behaviors of the resultant products. We found that the amount of microaggregation domains of POSS decreased, while the nano ones increased, when BCP content increased. All the aggregation domains were distributed in epoxy matrix uniformly at nanoscale with the addition of 10 phr BCP and 5 phr POSS monomers. The results indicated that BCP could effectively improve the compatibility between epoxy resin and POSS owing to its amphiphilicity in DGEBA. The fracture behavior of products transformed from brittle fracture to ductile fracture gradually with the increase of BCP, whereas the T g and E′ decreased.
Information
- Type
- Articles
- Information
- Copyright
- Copyright © Materials Research Society 2015
Footnotes
Contributing Editor: Linda S. Schadler
References
REFERENCES
- 4
- Cited by

