Article contents
Electrochemical and kinetic performance of amorphous/nanostructured TiNi-based intermetallic compound with Nb substitution synthesized by mechanical alloying
Published online by Cambridge University Press: 23 July 2018
Abstract
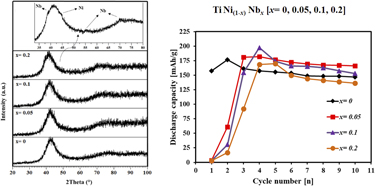
The electrochemical behavior of TiNi(1−x)Nbx (x = 0, 0.05, 0.1, 0.2) ternary intermetallic compounds synthesized by mechanical alloying was investigated and compared to that of binary TiNi. The structure of 20-h milled product with initial stoichiometric composition of TiNi0.95Nb0.05 was found to be amorphous/nanostructured. Upon cycling, this ternary milled product exhibited the highest discharge capacity (166.1 mA h/g) after 10 cycles and best cycle stability (∼91%) while those of the binary TiNi were 147 mA h/g and ∼83%, respectively; i.e., slight amount of Nb substitution (0.05 mol) for Ni in the TiNi not only increased discharge capacity and cycle stability but also enhanced the kinetics of hydrogen absorption/desorption through increasing the exchange current density and hydrogen diffusion coefficient. However, additional Nb content was found to have negative effect on electrochemical properties; this was related to the existence of Nb element in addition to the ternary amorphous/nanocrystalline structures.
Information
- Type
- Article
- Information
- Copyright
- Copyright © Materials Research Society 2018
References
REFERENCES
- 3
- Cited by

