Published online by Cambridge University Press: 24 May 2011
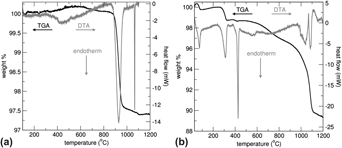
La4LiAuO8 is a stable Au3+ oxide that was recently examined as a possible model compound for the role of Au3+ in heterogeneous catalysis. Due to the paucity of thermodynamic data, the energetics of La4LiAuO8 and its likely decomposition product, LiLaO2, were investigated. The ΔHf−ox, of La4LiAuO8 and LaLiO2 are both exothermic at −187.7 ± 5.8 and −41.4 ± 9.6 kJ/mol, respectively. From the thermodynamic data, the decomposition temperature of La4LiAuO8 was calculated as either 979 ± 95 or 1331 ± 43 °C for the formation of LiLaO2 or Li2O, respectively. Thus, LiLaO2 is the expected decomposition product.