No CrossRef data available.
Published online by Cambridge University Press: 10 November 2014
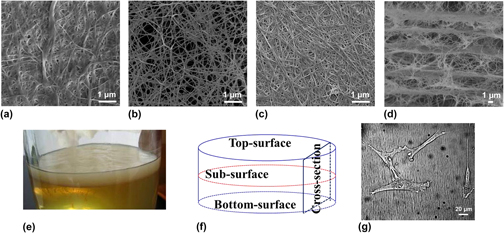
From the view of tissue engineering, the deficiency in porosity has impeded further application of bacterial cellulose (BC) as a super biomaterial. In this study, we used a combination method consisting of acetic acid treatment and freeze-drying operation to improve the porous profile of BC, as well as a simple and fast method to measure the thickness, density, and porosity of BC. Results have shown a significant improvement in the porosity of the inner structure of BC treated with acetic acid and freeze-drying. Microscopic observation by scanning electron microscopy exhibited explicit evidences that more orderly porous layer-by-layer structures and more pores were formed along the cross section of modified BC as compared with the control. The enhancement of mechanical properties and crystallinity of modified BC was also demonstrated due to the improvement of material porosity in the particular extent from 50.3 to 76.43%. Cell culture of human fibroblast cells exhibited good cell viability on modified BC, suggesting that a better porous profile of BC on the surface and cross section helps facilitate cells to attach, as well as potentially promotes cells to grow in. These significant results may open the possibility of producing BC nanomaterials for tissue engineering with desirable properties.