Article contents
Engineering star-shaped lactic acid oligomers to develop novel functional adhesives
Published online by Cambridge University Press: 17 April 2018
Abstract
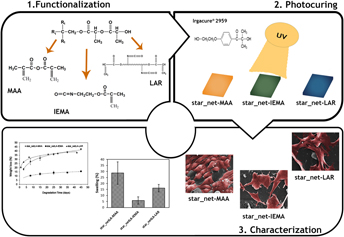
Direct polycondensation of L-lactic acid with a comonomer allows tailoring the properties of the product from the very first step. The viscous L-lactic acid co-oligomers with star-shaped architectures obtained were modified with three different acrylate monomers. Regardless the functionalization agent, UV curing was fast and all materials were cell compatible and promoted cell adhesion. The physical properties of the three star-shaped films exhibited a consistent trend as swelling capacity, hydrolytic instability, and gel content decreased simultaneously. A higher network density increased crosslinking degree and gel content among the films with an isocyanate group. The methacrylic end group functionalized material, lowest molecular weight, consistently exhibited the higher hydrolytic instability. Comparison of physical properties of these films with the corresponding linear materials reported previously confirmed the influence of precursor molecular architecture on the final material. The methodology developed herein is prone to scale-up and lead to the industrial production of new bioadhesives.
- Type
- Article
- Information
- Copyright
- Copyright © Materials Research Society 2018
References
REFERENCES
- 7
- Cited by



