Article contents
The evolution of structure and properties of PNIPA/clay nanocomposite hydrogels with the freezing time in polymerization
Published online by Cambridge University Press: 31 March 2014
Abstract
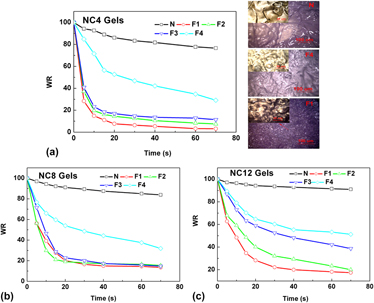
To prepare hydrogels with ultrarapid response rate and excellent mechanical properties, the poly(N-isopropylacrylamide)/clay nanocomposite hydrogels were synthesized by freezing polymerization technique. The start freezing time, as an important parameter determining the properties of gels, was designed and investigated. The results showed that the properties of gels including mechanical properties, swelling ratio, and swelling/deswelling rate were closely dependent on the freezing polymerization time. Comparably, the gels synthesized with earlier freezing time exhibit a faster swelling rate and an ultrarapid deswelling rate due to the integral interconnecting porous structure, while the swelling ratio, tensile strength and modulus decrease considerably. With the delay of start freezing time, the response rate decreases while the mechanical properties improve. Through the analysis of scanning electron microscope, differential scanning calorimetry, x-ray diffraction, dynamic rheological tests, and mechanical tests, the relevance of gels' structure with the freezing time was explored. It is reasonably presumed that freezing process impacts the effective crosslink of polymer chains by clay significantly, the earlier the freezing started, the more chains with free end existed in gels.
Information
- Type
- Articles
- Information
- Copyright
- Copyright © Materials Research Society 2014
References
REFERENCES
- 12
- Cited by

