Published online by Cambridge University Press: 12 March 2019
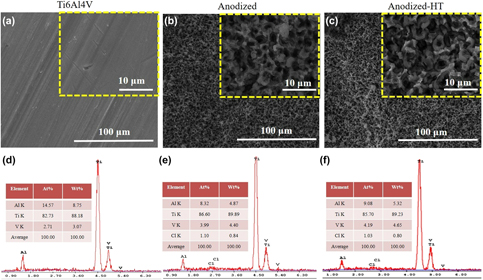
Ti6Al4V alloy is commonly used in hip and knee replacements due to its high strength, ductility, wear, and corrosion resistance. Despite its optimal physical and chemical properties, Ti6Al4V based orthopedic implants have a limited lifetime of only 15–20 years. One of the main reasons for having limited lifetime is the suboptimal integration of Ti6Al4V implants with the juxtaposed bone tissue (osseointegration). To enhance osseointegration, and thus prolong the lifetime of orthopedic implants, Ti6Al4V implants surfaces were modified to have bioactive properties using electrochemical anodization process. In this work, oxide based micropit structures were fabricated on Ti6Al4V surfaces using a fluoride-free electrolyte consisting of NH4Cl in distilled water. Micropit structures were characterized for their surface morphology, crystallinity, and chemistry before and after high temperature crystallization heat treatment. Upon interaction of Ti6Al4V samples with simulated body fluid up to 30 days, enhanced calcium phosphate mineral deposition was observed on anodized surfaces.