Article contents
Fabrication of nitrogen doped carbon encapsulated ZnO particle and its application in a lithium ion conversion supercapacitor
Published online by Cambridge University Press: 10 January 2017
Abstract
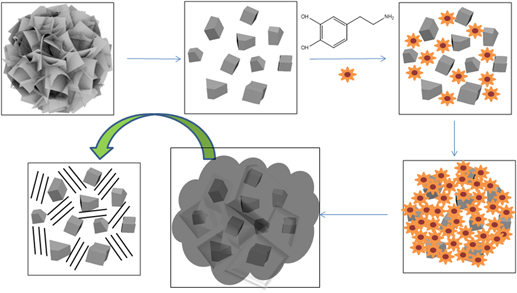
A new lithium ion hybrid supercapacitor is reported, in which the negative electrode was made from ZnO nano-crystals coated with a nitrogen doped carbon, and a positive electrode composed of activated carbon. The ZnO nano-crystals were highly dispersed in a nitrogen doped carbon matrix through a bio-inspired route. Dopamine, used as the nitrogen and carbon source, self-polymerized and deposited onto the surface of ZnO nano-crystal. After pyrolysis, a nitrogen doped amorphous carbon coated ZnO nano-crystal materials were obtained. The characteristics of the synthesized carbon coated ZnO nano-crystal electrode as well as the electrochemical performance of the hybrid device were investigated. The ZnO nano-crystal structure was preserved in the course of the carbon coating. The lithium ion supercapacitor demonstrated a high capacity and good cycling stability. Such good performance can be attributed to improved conductivity, the prevention of ZnO nano particles from pulverization and the high degree of crystallinity of the ZnO material.
Keywords
- Type
- Articles
- Information
- Copyright
- Copyright © Materials Research Society 2017
References
REFERENCES
- 8
- Cited by



