Published online by Cambridge University Press: 12 November 2020
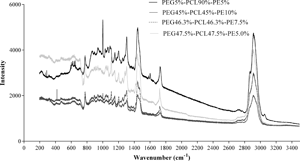
Interactions between smooth muscle cells (SMCs) and biomaterials must not result in phenotype changes as this may generate uncontrolled multiplication processes and occlusions in vascular grafts. The aim of this study was to relate the hydrolytic stability and biocompatibility of polyurethanes (PUs) on SMCs. A higher polycaprolactone (PCL) concentration was found to improve the hydrolytic stability of the material and the adhesion of SMCs. A material with 5% polyethylene glycol, 90% PCL, and 5% pentaerythritol presented high cell viability and adhesion, suggesting a contractile phenotype in SMCs depending on the morphology. Nevertheless, all PUs retained their elastic modulus over 120 days, similar to the collagen of native arteries (~10 MPa). Furthermore, aortic SMCs did not present toxicity (viability over 80%) and demonstrated adherence without any abnormal cell multiplication processes, which is ideal for the function to be fulfiled in situ in the vascular grafts.