Article contents
Influence of interactions between β′ precipitates and long period stacking ordered structures on corrosion behaviors of Mg–10Gd–5Y–2Zn–0.5Zr (wt%) alloy
Published online by Cambridge University Press: 10 December 2017
Abstract
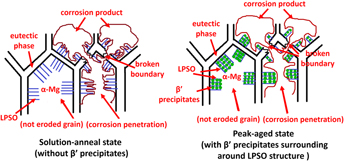
In this paper, corrosion behaviors of Mg–10Gd–5Y–2Zn–0.5Zr (wt%) alloy (GWZ1052K) in different aging stages are investigated using immersion tests and electrochemical measurements in 3.5 wt% NaCl aqueous solution. The corrosion resistance is found to increase from the solution-anneal to peak-aged condition, which is attributed to microstructure evolutions of β′ precipitates and nearly unchanged long period stacking ordered (LPSO) structures. The broken network LPSO structures no more act as corrosion barriers, thus inversely worsening the galvanic corrosion. β′ precipitates uniformly surround the LPSO lamellas, those partly enhancing corrosion resistance. The potentiodynamic polarization curves also show the best corrosion resistance in the peak-aged stage, suggesting the similar tendency of corrosion behaviors. And the results of electrochemical impedance spectrum are consistent with the morphology of the corrosion surface. Further equivalent circuit is established to investigate the corrosion mechanism.
Information
- Type
- Articles
- Information
- Copyright
- Copyright © Materials Research Society 2017
Footnotes
Contributing Editor: Jürgen Eckert
References
REFERENCES
- 6
- Cited by


