Published online by Cambridge University Press: 15 May 2017
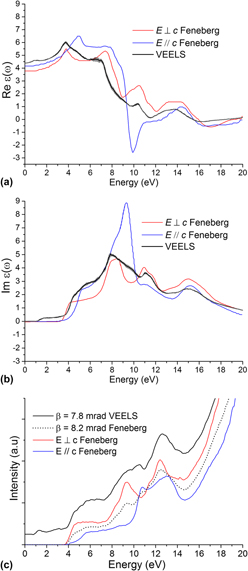
For the first time, valence electron energy-loss spectroscopy (VEELS) was applied to individual single-crystalline SnO2 nanowires to investigate the dielectric function, band gap, and optical absorption coefficient. The results are compared with data from optical techniques such as spectroscopic ellipsometry and UV-Vis, and theoretical calculations from variations of density functional theory. The data obtained agree well with the standard optical and theoretical techniques. The dielectric function and optical absorption coefficient are given up to 20 eV, which otherwise requires a synchrotron source and large single crystals via optical methods. The energy loss function is given up to 40 eV, which gives a useful comparison to previous theoretical studies in an energy range that cannot be achieved via optical measurements. The comparison gives confidence in the accuracy of this method for exploring spatially-resolved measurements in individual nanoparticles or more complex nanostructures that are otherwise difficult to measure accurately using optical techniques.
Contributing Editor: Gary L. Messing