Published online by Cambridge University Press: 03 March 2011
The high temperature structural stability of nanograined NiCr alloys reinforced with nanoscale yttria and alumina dispersoids, fabricated by electron beam physical vapor deposition (EBPVD), was examined. The yttria particles coarsened very little and also inhibited grain growth in the matrix successfully, whereas the alumina dispersoids coarsened rapidly and were not as effective in restricting matrix grain growth. A hierarchy of phase transformations took place in the Al2O3 particles present as nano dispersoids in a nanograined NiCr matrix 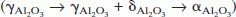 , on annealing. Coarsening of the alumina particles was accompanied by these phase transitions. The phase evolution is attributed to differences in free energies between the metastable and stable phases and a kinetic hierarchy in nucleation, brought about by structural and hence interfacial energy considerations.
, on annealing. Coarsening of the alumina particles was accompanied by these phase transitions. The phase evolution is attributed to differences in free energies between the metastable and stable phases and a kinetic hierarchy in nucleation, brought about by structural and hence interfacial energy considerations.