Published online by Cambridge University Press: 24 June 2020
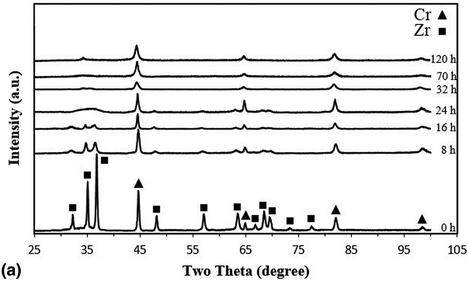
In this research, the mechanical alloying (MA) technique was used to study solid solubility in the immiscible Zr–Cr alloy system. At first, Zr and Cr powders were milled, and then, the phase evolution, alloying mechanism, microstructural change, and mechanical properties of the milled powders were investigated by X-ray diffraction technique, scanning electron microscopy along with energy dispersive spectroscopy, transition electron microscopy, and microhardness measurements. Moreover, the solubility limit of Zr in Cr lattice was obtained by Vegard's law. The results showed that the MA was significantly enhanced the solubility of Zr in Cr up to about 21.6 wt% after an optimum milling time of 32 h and led to form an amorphous/nanocrystalline composite of Zr-reach and Cr-reach supersaturated solid solutions with the microhardness value of 503 Hv approximately. Also, the thermodynamic analysis indicated that the Gibbs free energy changes for the amorphous and solid solution were positive, which were provided by the MA process.