Article contents
Microstructure and compressive properties of porous Ti–Nb–Ta–Zr alloy for orthopedic applications
Published online by Cambridge University Press: 05 December 2019
Abstract
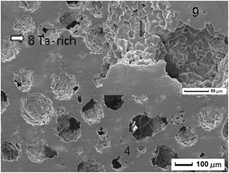
In recent years, there has been a significant thrust toward the development of novel implant alloys based on β-Ti with low Young’s modulus to prevent stress shielding. In this study, porous Ti–Nb–Ta–Zr alloys with porosity of <55% and macro-pore size of 100–400 μm for biomedical applications were successfully fabricated by a space-holder method. The microstructure and compressive behavior were studied. The results show that the micro-pore size of porous Ti–Nb–Ta–Zr alloys decreases with an increase in the amount of the process control agent (PCA), which has no obvious effect on the porosity and the macro-pore size formed by the space holder. Porous Ti–Nb–Ta–Zr alloys fail mainly because of the cleavage and ductile fracture with some dimples in compression. The compressive modulus increases from 0.6 to 6.5 GPa with the increase in the PCA and decrease of the space holder. The influence mechanism has been analyzed by the finite element calculation and the Gibson–Ashby model.
Keywords
Information
- Type
- Article
- Information
- Copyright
- Copyright © Materials Research Society 2019
References
- 5
- Cited by

