Published online by Cambridge University Press: 13 November 2013
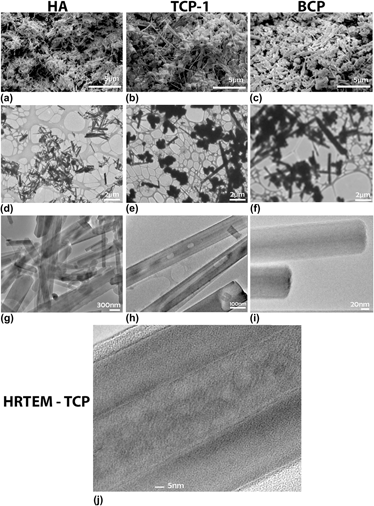
Calcium phosphates (CaPs) are major chemical constituents of mammalian bone. Their osteoconductivity in vitro and in vivo has encouraged their use in biomaterial applications such as implant materials and drug delivery. High aspect ratio nanoparticles are attractive for many biomedical applications; however, precise control of the phase and morphology is challenging. The impact of fuel-to-oxidant ratio, pH, and cation chemistry on morphology and phase was studied for CaP-based compositions by microwave-assisted solution combustion synthesis (MASCS) in a urea–nitrate (fuel–oxidant) system. An initial calcium to phosphate ratio of 1.5 was used. Highly crystalline hydroxyapatite (HA) and biphasic CaP nanoparticle compositions were produced as confirmed by x-ray diffraction, scanning electron microscopy, and transmission electron microscopy. MASCS was capable of synthesizing high aspect ratio (∼5 to 20) single and biphasic CaP nanoparticles with diameters ranging from 250 to 500 nm and lengths between 2 and 10 μm.
To send this article to your Kindle, first ensure no-reply@cambridge.org is added to your Approved Personal Document E-mail List under your Personal Document Settings on the Manage Your Content and Devices page of your Amazon account. Then enter the ‘name’ part of your Kindle email address below. Find out more about sending to your Kindle. Find out more about saving to your Kindle.
Note you can select to save to either the @free.kindle.com or @kindle.com variations. ‘@free.kindle.com’ emails are free but can only be saved to your device when it is connected to wi-fi. ‘@kindle.com’ emails can be delivered even when you are not connected to wi-fi, but note that service fees apply.
Find out more about the Kindle Personal Document Service.
To save this article to your Dropbox account, please select one or more formats and confirm that you agree to abide by our usage policies. If this is the first time you used this feature, you will be asked to authorise Cambridge Core to connect with your Dropbox account. Find out more about saving content to Dropbox.
To save this article to your Google Drive account, please select one or more formats and confirm that you agree to abide by our usage policies. If this is the first time you used this feature, you will be asked to authorise Cambridge Core to connect with your Google Drive account. Find out more about saving content to Google Drive.