Published online by Cambridge University Press: 08 February 2019
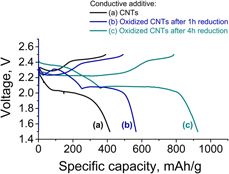
In this study, for the first time, chemically modified carbon nanotubes (CNTs) were used as a conductive additive in the cathode composite for lithium–sulfur batteries. Oxidation of pure CNTs has been carried out using modified Hummers’ method, and to partially remove oxygen groups from the CNT surface and increase their electronic conductivity, oxidized CNTs have been hydrothermally treated. The cathode slurry was mixed in water with a water-soluble LA133 binder. Despite the decrease in electronic conductivity of CNTs after chemical treatment, the presence of structural defects and oxygen groups provides uniform distribution of modified CNTs in the sulfur-based composite, which results in more than twice higher electrode specific capacity compared with the electrodes comprising pure CNTs. Using chemically modified CNTs as a conductive additive is proposed as an effective way for the preparation of nontoxic and cost-effective water-based cathode slurries in lithium–sulfur batteries.