Published online by Cambridge University Press: 17 July 2018
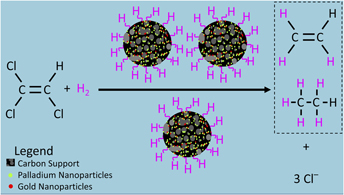
Palladium (Pd) and gold (Au) nanoparticles (NPs) hybridized on two types of carbon supports, graphene and granular activated carbon (GAC), were shown to be promising catalysts for the sustainable hydrodehalogenation of aqueous trichloroethylene (TCE). These catalysts are capable of degrading TCE more rapidly than commercial Pd-on-GAC catalysts. The catalysts were synthesized at room temperature without the use of any environmentally unfriendly chemicals. Pd was chosen for its catalytic potency to break down TCE, while Au acts as a strong promoter of the catalytic activity of Pd. The results indicate that both graphene and GAC are favorable supports for the NPs due to high surface-to-volume ratios, unique surface properties, and the prevention of NP aggregation. The properties of NP catalysts were characterized using electron microscopy and spectroscopy techniques. The TCE degradation results indicate that the GAC-supported catalysts have a higher rate of TCE removal than the commercial Pd-on-GAC catalyst, and the degradation rate is greatly increased when using graphene-supported samples.
Present address: Oak Ridge Institute for Science and Education Fellow, Office of Superfund Remediation and Technology Innovation, U.S. Environmental Protection Agency, Arlington, Virginia 22201, USA.