Published online by Cambridge University Press: 28 June 2011
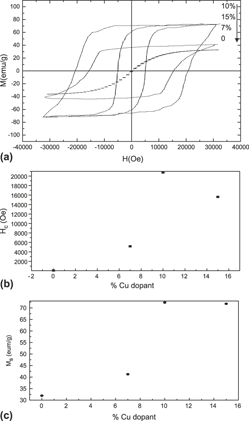
Iron oxides, including maghemite (γ-Fe2O3) and magnetite (Fe3O4), have been widely applied in many fields. For technological advances in the future, further improvements of their ferromagnetic properties are desirable. The development of iron ferrites with a large coercive field (Hc) is one of issues of consequence. For ferrites, however, enlarging the Hc value is not easy because of their low magnetocrystalling anisotropy constant. Here we report single-crystalline Cu-doped γ-Fe2O3 nanowires in which the controlled diameter (70–100 nm) and the graded Cu dopant (7, 10, and 15%) are directly obtained by a simple chemical vapor deposition technique. In particular, the coercive value (over 2 T) of 10% Cu-doped γ-Fe2O3 nanowires is much higher than that (<80 Oe) of undoped γ-Fe2O3 nanowires at room temperature. On the basis of the experimental magnetization data, the achievement of such a higher coercive field of Cu-doped γ-Fe2O3 (10%) nanowires is tentatively suggested.