Published online by Cambridge University Press: 12 February 2015
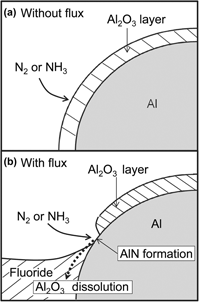
The production of aluminum nitride (AlN) from aluminum metal was investigated in this study. The nitridation of Al (in rod, powder, and thin-plate forms) was facilitated by dissolving the Al2O3 thin films formed on the Al samples with a molten fluoride mixture (KF–45 mol% AlF3, KF, or LiF–50 mol% KF). AlN was formed when NH3 gas was supplied to the Al sample (in both solid and liquid forms) wetted by molten fluoride mixture. The lowest temperature at which AlN was successfully produced was 773 K. No AlN was formed when N2 or H2–25% N2 gas was supplied to the Al sample, even when a molten fluoride mixture was used. The reaction rate for the nitridation of Al powder increased with the temperature and reached 99% after 3 h at 1173 K. AlN thin films of 2–5 μm thickness were formed on Al thin plates (0.075–1.0 mm thick) at 873 K.