Published online by Cambridge University Press: 06 November 2013
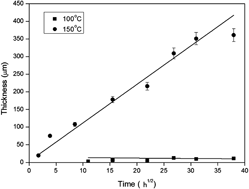
Interfacial reactions at 100 and 150 °C in the Sn–20.48 at.% In–3.05 at.% Ag (Sn–20.0 wt% In–2.8 wt% Ag)/Ni couples are studied. Three unusual phenomena are observed. First, liquation is found in Sn–20.48 at.% In–3.05 at.% Ag (Sn–In–Ag)/Ni couples that are reacted at 150 °C, which is lower than the melting points of both the solder and the Ni substrate. In addition to the Ni3Sn4 phase, liquid phase is formed in the reaction layer. Second, the liquid phase disappears and isothermal solidification occurs when there is prolonged isothermal heat treatment at 150 °C. The results are similar to those for transient liquid phase bonding. Third, the thickness of the reaction layer in Sn–In–Ag/Ni couples that are reacted for 1440 h at 150 °C is 40 times thicker than that of those reacted at 100 °C. The reaction mechanisms for these three unusual phenomena: liquation, isothermal solidification, and an extraordinary increase in the reaction rate for only 50 °C difference in temperature are elaborated and are related to each other.