Article contents
Rheological phase reaction method synthesis and characterizations of xLiMn0.5Fe0.5PO4–yLi3V2(PO4)3/C composites as cathode materials for lithium ion batteries
Published online by Cambridge University Press: 28 November 2019
Abstract
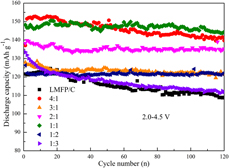
A series of xLiMn0.5Fe0.5PO4–yLi3V2(PO4)3/C (x:y= 4:1, 3:1, 2:1, 1:1, 1:2, and 1:3) composite cathode materials for lithium-ion batteries are successfully prepared by the rheological phase reaction method. The structures, morphologies, and electrochemical properties of these composite materials are studied. The results indicate that xLiMn0.5Fe0.5PO4–yLi3V2(PO4)3/C composites are composed of LiMn0.5Fe0.5PO4 and Li3V2(PO4)3 phases and mutual doping exists. The initial discharge capacities, initial Coulombic efficiencies, and capacity retentions of composites increase but then decline with the increase of Li3V2(PO4)3 content. All the composites show higher capacity retentions than LiMn0.5Fe0.5PO4/C and Li3V2(PO4)3/C single phases except LMFP–3LVP/C. The composite material of x:y= 1:1 exhibits remarkably superior electrochemical performance than the single phases and other composites both in discharge capacity and cycle performance, delivering the initial discharge capacity of 148.2 mA h/g (2.0–4.5 V) and 170.1 mA h/g (2.0–4.8 V) at 0.1 C. And the corresponding capacity retentions are 98.0 and 90.4% after 100 cycles, respectively.
Keywords
Information
- Type
- Invited Paper
- Information
- Journal of Materials Research , Volume 35 , Issue 1: Focus Section: Advances in Battery Technology: Material Innovations in Design and Fabrication , 14 January 2020 , pp. 2 - 11
- Copyright
- Copyright © Materials Research Society 2019
References
- 3
- Cited by

