Crossref Citations
This article has been cited by the following publications. This list is generated based on data provided by
Crossref.
He, Jun-fei
Xiong, Zhi-bo
Du, Yan-ping
Lu, Wei
and
Tian, Su-le
2021.
Morphology effect of tungsten oxide on Ce/W catalyst for selective catalytic reduction of NO with NH3: Influence of structure-directing agents.
Journal of the Energy Institute,
Vol. 94,
Issue. ,
p.
85.
Chen, Jiazhe
Guo, Luyao
Zhu, Hongchang
Qiu, Yu
Yin, Dejia
Zhang, Tao
Chen, Jianjun
Peng, Yue
and
Li, Junhua
2021.
Balancing redox and acidic properties for optimizing catalytic performance of SCR catalysts: A case study of nanopolyhedron CeO -supported WO.
Journal of Environmental Chemical Engineering,
Vol. 9,
Issue. 5,
p.
105828.
Liu, Yuanyuan
Gao, Fengyu
Ko, Songjin
Wang, Chengzhi
Liu, Hengheng
Tang, Xiaolong
Yi, Honghong
and
Zhou, Yuansong
2022.
Superior catalytic performance within H2O-vapor of W-modified CoMn2O4/TiO2 catalyst for selective catalytic reduction of NOx with NH3.
Chemical Engineering Journal,
Vol. 434,
Issue. ,
p.
134770.
Xiong, Zhi-bo
Guo, Fu-cheng
Zhang, Jia-xin
Lu, Wei
and
Shi, Huan-cong
2022.
Influence of g-C3N4 doping on the NH3-SCR activity of Cerium–tungsten–titanium mixed oxide catalyst.
Journal of Materials Research,
Vol. 37,
Issue. 3,
p.
835.
Xiong, Zhibo
Wang, Wei
Li, Jun
Huang, Lihao
and
Lu, Wei
2022.
The synergistic promotional effect of W doping and sulfate modification on the NH3-SCR activity of CeO2 catalyst.
Molecular Catalysis,
Vol. 522,
Issue. ,
p.
112250.
Xie, Baocheng
Wei, Zijian
Ding, Min
Gao, Meixingzi
Wang, Li
Zhan, Wangcheng
Dai, Qiguang
Guo, Yun
Wang, Aiyong
and
Guo, Yanglong
2024.
Enhanced Ru-O-Ce interaction by solid solution structure of Ru-CexZr1-xO2 catalysts for efficient catalytic combustion of vinyl chloride.
Applied Catalysis B: Environment and Energy,
Vol. 350,
Issue. ,
p.
123926.
Wang, Wei
Xu, Wenying
Zhao, Zhiwei
Cheng, Mingyan
Xun, Menghan
and
Liu, Huimin
2024.
Method and Application of Surface Modification of Cerium Dioxide.
Advanced Engineering Materials,
Vol. 26,
Issue. 14,
Lu, Muyu
Gao, Fengyu
Tan, Yiran
Yi, Honghong
Gui, Yang
Xu, Yan
Wang, Ya
Zhou, Yuansong
Tang, Xiaolong
and
Chen, Linjiang
2024.
Knowledge-Driven Experimental Discovery of Ce-Based Metal Oxide Composites for Selective Catalytic Reduction of NOx with NH3 through Interpretable Machine Learning.
ACS Applied Materials & Interfaces,
Vol. 16,
Issue. 3,
p.
3593.
Luo, Ning
Gao, Fengyu
Liu, Hengheng
Xiong, Tingkai
Wen, Jiajun
Duan, Erhong
Wang, Chengzhi
Zhao, Shunzheng
Yi, Honghong
and
Tang, Xiaolong
2024.
Hierarchical structured Ti-doped CeO2 stabilized CoMn2O4 for enhancing the low-temperature NH3-SCR performance within highly H2O and SO2 resistance.
Applied Catalysis B: Environmental,
Vol. 343,
Issue. ,
p.
123442.
Zhou, Gang
Zhang, Jinhao
Xin, Yueying
Zhang, Yongliang
Hu, Shengyong
and
Zhang, Xinyuan
2025.
High-performance Ce-modified CoMnOx catalysts for NH3-SCR: NOx catalytic performance and sulfur tolerance.
Applied Surface Science,
Vol. 693,
Issue. ,
p.
162775.
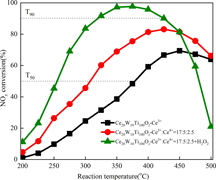
 $\lpar {\rm {O}^{\prime}}_{\rm \alpha} \rpar$ due to the activation of chemisorbed water on the surface of the catalyst. The addition of Ce4+ and H2O2 shows a synergistic promotional effect, which is due to the largest BET surface area and the highest concentrations of Oα or/and
$\lpar {\rm {O}^{\prime}}_{\rm \alpha} \rpar$ due to the activation of chemisorbed water on the surface of the catalyst. The addition of Ce4+ and H2O2 shows a synergistic promotional effect, which is due to the largest BET surface area and the highest concentrations of Oα or/and  ${\rm {O}^{\prime}}_{\rm \alpha}$. Ce20W10Ti100Oz–Ce3+:Ce4+ = 17.5:2.5 + H2O2 exhibits the highest catalytic activity compared with the conventional ones (Fig. 5).
${\rm {O}^{\prime}}_{\rm \alpha}$. Ce20W10Ti100Oz–Ce3+:Ce4+ = 17.5:2.5 + H2O2 exhibits the highest catalytic activity compared with the conventional ones (Fig. 5).

