Published online by Cambridge University Press: 10 September 2020
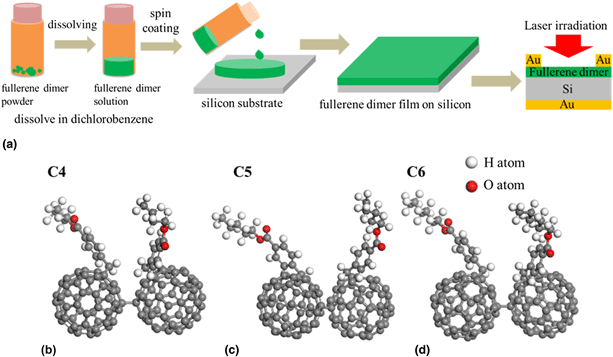
Fullerene dimers have attracted extensive attention due to their unique structures and fascinating properties. Here, fullerene dimer derivatives with four to six carbon atoms in the esters are designed and synthesized. The property differences that caused by the carbon number in the esters of the fullerene dimers are investigated by performing their electrochemical, optical, and photoelectric measurements. As the carbon atom numbers in the esters increase from four to five and six, the absorption intensities increase to 1.6- and 4.4-folds. The intensities of the fluorescence spectra increase to 1.8- and 5.2-folds. Their photocurrent increases to 2- and 7-folds under the irradiation of a 405-nm laser. The LUMO energy levels move downward slightly from −3.89 to −3.90 and −3.92 eV, respectively. Our results indicate that as the carbon number increases, the carbon chain lengths in the ester structures increase, very slight effects produced on the energy levels of the fullerene dimers, but strongly contribute to their chemical activities and thus the photoelectronic efficiencies.
These authors contributed equally to this paper.