Published online by Cambridge University Press: 22 December 2015
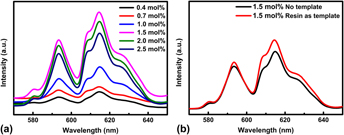
ZrO2:Eu3+ hollow spheres were successfully fabricated with the resin microspheres as the template. The sample characterizations were carried out by means of x-ray diffraction (XRD), scanning electron microscope (SEM), and photoluminescence spectra. XRD results revealed that Eu3+-doped samples were pure t-ZrO2 phase after being calcined at 873 K. SEM results exhibited that this Eu3+ doped ZrO2 was hollow spheres; the diameter and thickness of which were about 450 and 50 nm, respectively. Upon excitation at 394 nm, the orange-red emission bands at the wave length longer than 570 nm were from 5D0 → 7FJ (J = 1, 2) transitions. The asymmetry ratio of (5D0 → 7F2)/(5D0 → 7F1) intensity is about 1.61, 1.26, 1.42, 1.42, 1.40, and 1.38 for the Eu3+ concentration 0.4, 0.7, 1.0, 1.5, 2.0, and 2.5 mol%, respectively. These values suggest that the asymmetry ratio of Eu3+ ions is independent of the doping concentration. The optimal doping concentration of Eu3+ ions in ZrO2 is 1.5 mol%. According to Dexter's theory, the critical distance between Eu3+ ions for energy transfer was determined to be 16 Å.
Co-first author
Contributing Editor: Xiaobo Chen