Article contents
Systematic dependence of kinetic and thermodynamic barriers to homogeneous silica nucleation on NaCl and amino acids
Published online by Cambridge University Press: 15 February 2019
Abstract
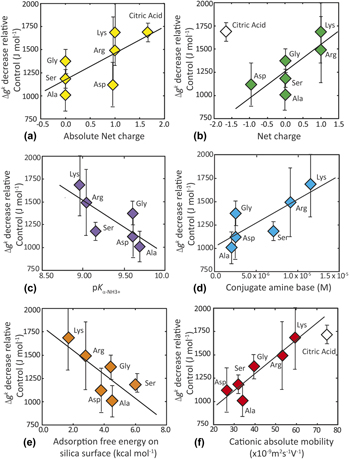
The kinetics of silica polymerization was measured in silicic acid solutions containing a suite of 0.1 M amino acids, 0.1 M citric acid, 0.7 M NaCl, and 0.10 M NaCl (Control). Fitting a modified classical rate model to measurements of induction time (τ) at 20 °C for a series of supersaturated solutions, we estimate the thermodynamic barrier (ΔGc), interfacial free energy (γ), and kinetic barrier (Δagk) for silica nucleation. For 0.10 M NaCl solutions, γControl = 54.9 ± 1.6 mJ/m2 and ΔagkControl = 2.29 × 10−19 J/mol. These values are consistent with previous reports for amorphous and fused silica materials. To facilitate comparisons with the treatments, ΔagkControl is converted to a molar basis and used as a reference datum, such that ΔagkControl = 0.0 J/mol. The effects of salt and organic acids on nucleation rate have thermodynamic and kinetic origins, respectively. Faster nucleation rates measured in 0.7 M NaCl solutions arise from a lower interfacial free energy, such that γ0.7 M NaCl = 51.4 ± 1.7 mJ/m2. Organic acids increase rate through biomolecule-specific reductions in Δagk. Catalytic effects are greatest for lysine (Δagklysine = −1685 ± 315) and citric acid (Δagkcitric = −1690 ± 96 J/mol). Reductions in the kinetic barrier correlate with net positive charge of the amino acids and dissociation of the amine  $\left( {{K_{\alpha {\rm{ ‐ N}}{{\rm{H}}_3}^ {\bf{+}} }}} \right)$ group and thus the abundance of the conjugate base. Citric acid, lacking amine groups, promotes the greatest rate enhancement, thus demonstrating the role(s) of additional kinetic factors in promoting nucleation rate. Catalytic activity correlates with multiple physical and chemical properties of the organic acids.
$\left( {{K_{\alpha {\rm{ ‐ N}}{{\rm{H}}_3}^ {\bf{+}} }}} \right)$ group and thus the abundance of the conjugate base. Citric acid, lacking amine groups, promotes the greatest rate enhancement, thus demonstrating the role(s) of additional kinetic factors in promoting nucleation rate. Catalytic activity correlates with multiple physical and chemical properties of the organic acids.
- Type
- Invited Article
- Information
- Journal of Materials Research , Volume 34 , Issue 3: Focus Issue: Understanding Water-Oxide Interfaces to Harness New Processes and Technologies , 14 February 2019 , pp. 442 - 455
- Copyright
- Copyright © Materials Research Society 2019
References
- 15
- Cited by


