Published online by Cambridge University Press: 15 April 2016
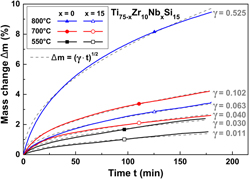
The glass-forming Ti75Zr10Si15 and Ti60Zr10Nb15Si15 alloys composed of nontoxic elements may represent new materials for biomedical applications. For this study, melt-spun alloy samples exhibiting glass–matrix nanocomposite structures were subjected to thermal oxidation treatments in synthetic air to improve their surface characteristics. 550 °C was identified as the most appropriate temperature to carry out oxidative surface modifications while preserving the initial metastable microstructure. The modified surfaces were evaluated considering morphological and structural aspects, and it was found that the oxide films formed at 550 °C are amorphous and consist mainly of TiO2; their thicknesses were estimated to be ∼560 nm for Ti75Zr10Si15 and ∼460 nm for Ti60Zr10Nb15Si15. The thermally treated sample surfaces exhibit not only higher roughnesses and higher hardnesses but also improved wettability compared to the as-spun materials. By immersion of oxidized samples in simulated body fluid Ca- and P-containing coatings exhibiting typical morphologies of apatite are formed.