No CrossRef data available.
Published online by Cambridge University Press: 08 March 2017
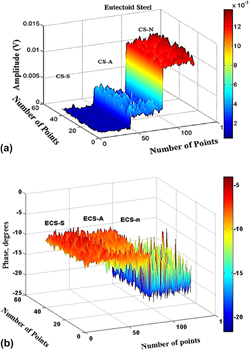
A eutectoid carbon steel was studied at three different annealing heat treatment cycles: spheroidizing, isothermal annealing, and normalization (air cooling). The aim of this study was to determine the correlation among thermal, structural, and metallurgical properties, as a result of the annealing heat treatment. Microstructure differences were produced by the heat treatment cooling rate with significant effects on Vickers nanohardness, thermal properties, and crystallinity. It was reflected in photothermal radiometry (PTR) images as in thermal conductivity and diffusivity. The amplitude signal increased as the cooling rate increased. It means that as the cooling rate increased, crystallinity, thermal diffusivity, and conductivity decreased. The cooling rate affected the metallurgical structure directly, and consequently, the nanohardness which decreased due to the solid solution formation and decomposition of the pearlite phase. As the cooling rate increased, the nanohardness increased modifying structural properties and the steel crystallinity. As the cooling rate decreased, the crystallinity increased.
Contributing Editor: Jürgen Eckert