Published online by Cambridge University Press: 13 April 2017
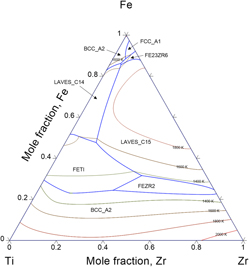
Modern hydrogen technology requires materials with high capacity storage. Many metals and alloys form cheap metal hydrides that can contain a high volume density of hydrogen. The quaternary alloy Cr–Fe–Ti–Zr with Laves phases is one promising hydrogen storage material. Understanding phase equilibria properties is essential to improve the Laves phases’ hydrogen storage capacity. In this work, the thermodynamic description of two constituent ternary phase materials, Cr–Ti–Zr and Fe–Ti–Zr are investigated using the Calphad method. A set of Gibbs energies was optimized during this work and good agreement between modeling and available experimental information was found. Moreover, a new thermodynamic model for a binary Fe–Zr system was developed based on recent experimental investigation about intermetallic compounds FeZr2 and FeZr3. Obtained in this work results can find application in development of new hydrogen storage materials.
Contributing Editor: Jürgen Eckert