Published online by Cambridge University Press: 16 July 2019
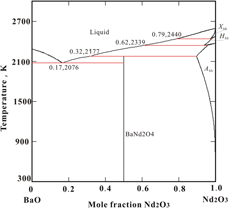
The BaNd2O4 compound and the BaO–Nd2O3 system are of interest for electroceramics. In this work, we synthesized BaNd2O4 by solid-state reaction, measured its heat capacity from 573 to 1273 K by differential scanning calorimetry, and determined its enthalpy of formation from component oxides at 298 K by high-temperature oxide melt solution calorimetry to be −43.75 ± 4.68 kJ/mol. Our newly determined values and available literature data were employed to assess the phase equilibria in the BaO–Nd2O3 system using CALPHAD methodology. A self-consistent thermodynamic database and the calculated phase diagram of the BaO–Nd2O3 system are provided. The optimized thermodynamic parameters are in good agreement with available experimental data. BaNd2O4 is calculated to melt incongruently at 2177 K. This thermodynamic analysis is essential for the optimization of synthesis conditions for materials and for the evaluation of their stability under appropriate technological operating conditions.
This author was an editor of this journal during the review and decision stage. For the JMR policy on review and publication of manuscripts authored by editors, please refer to http://www.mrs.org/editor-manuscripts/.