Article contents
The thermophysical properties and defect chemistry of HfO2–Sm3TaO7 ceramics
Published online by Cambridge University Press: 08 July 2020
Abstract
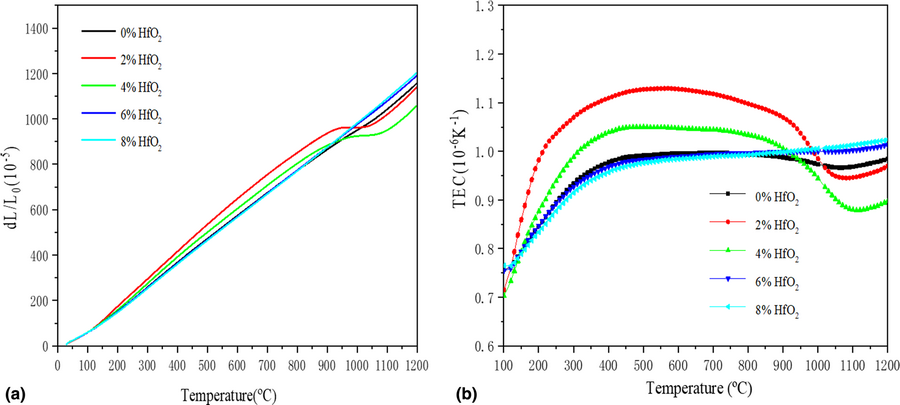
HfO2–Sm3TaO7 ceramics are prepared through a solid-state reaction method. The X-ray diffraction and structural refinement show that the phase structures of HfO2–Sm3TaO7 ceramics are an ordered orthorhombic phase and the space groups are belonging to Ccmm. The degree of the structural disorder increases with increasing HfO2 content. The solid solution mechanism reveals that Hf4+ exists in the form of interstitial ions that cause crystal expansion when the doping content is less than 4 mol%. When the doping concentration of HfO2 ≥ 4 mol%, the Hf4+ ions can substitute an equal number of Sm3+ and Ta5+ ions. The phase transition of Sm3TaO7 ceramics is removed with increasing HfO2 content, and the 8 mol% HfO2–Sm3TaO7 ceramics have a high thermal expansion coefficient of 10.2 × 10−6 K−1 at 1200 °C. The 2 mol% HfO2–Sm3TaO7 ceramics have the lowest thermal conductivity (1.03 W/m K at 900 °C), which is lower than previous research of the 7–8 YSZ. The outstanding thermophysical properties of HfO2–Sm3TaO7 ceramics indicate that they are potential thermal-barrier coating materials.
Information
- Type
- Article
- Information
- Copyright
- Copyright © Materials Research Society 2020
References
- 2
- Cited by


