Article contents
Three-dimensional porous layered double hydroxides growing on carbon cloth as binder-free electrodes for supercapacitors
Published online by Cambridge University Press: 13 June 2017
Abstract
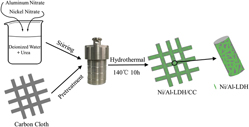
In this work, a three-dimensional (3D) porous hybrid nickel/aluminum layered double hydroxide (Ni/Al-LDH)-carbon cloth (CC), the working electrode without binders or conductive additions for supercapacitor, was successfully synthesized via facile one-step hydrothermal method. The as-obtained Ni/Al-LDH/CC sample exhibited good charge storage performance (the specific capacitance was up to 359 F/g at a current density of 0.3 A/g), as well as superior cycling stability (5.9% capacitance increase after 3000 cycles at 1.0 A/g). Furthermore, an asymmetric supercapacitor, Ni/Al-LDH/CC as positive electrode and activated carbon (AC) as negative electrode (Ni/Al-LDH/CC//AC), achieved a high energy density (20.9 Wh/kg vs. the power density 262.5 W/kg) and good cycle lifetime (83.9% retention of the initial value after 3000 cycle tests at a current density of 0.5 A/g). The unique 3D porous structure and binder-free electrode display great potential in supercapacitors.
Keywords
- Type
- Articles
- Information
- Copyright
- Copyright © Materials Research Society 2017
Footnotes
Contributing Editor: Scott T. Misture
References
REFERENCES
- 8
- Cited by



