Article contents
Tunable glass reference materials for quantitative backscattered electron imaging of mineralized tissues
Published online by Cambridge University Press: 21 August 2012
Abstract
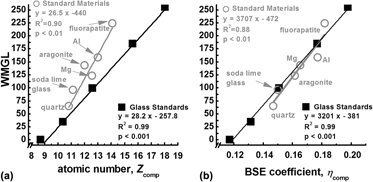
Backscattered electron microscopy provides gray-level contrast resulting from variations in atomic composition. Through the use of reference materials, quantitative backscattered electron (qBSE) imaging can be used to measure the mineral content of mineralized tissues at submicron resolution. We have developed novel tunable reference materials that can be adjusted for analysis of an individual tissue or a wide range of tissues with variable atomic density. As an alternative to conventional metallic reference materials, these amorphous materials maintain long-term stability and possess no long-range order that may induce channeling contrast. Using these reference materials, we characterized the mineral content of a broad range of mineralized tissues from immature mouse femur to whale bulla. Mineral volume fraction correlated to more traditional measurements of mineral content with microcomputed tomography and ashing techniques. Further, we demonstrate the advantage of location-matched measurements of nanomechanical properties and qBSE mineral content.
- Type
- Articles
- Information
- Copyright
- Copyright © Materials Research Society 2012
References
REFERENCES
- 5
- Cited by


