Introduction
Fisetin (3, 3′, 4′, 7-tetrahydroxyflavone; Fig. 1) is a flavonoid polyphenol found abundantly in various fruits and vegetables and has been recognised to have a broad spectrum of potential health benefits including chemoprevention, neuroprotection, cardioprotection, anti-aging and cellular senescence(Reference Arai, Watanabe and Kimira1–Reference Mehta, Pawar and Mahadik3). Several in vitro and in vivo studies have successfully demonstrated the anti-cancer effects through various mechanisms such as inducing apoptosis and autophagy, cell cycle arrest, inhibiting metastasis and angiogenesis, as well as anti-inflammatory and antioxidant activities(Reference Kashyap, Garg and Tuli4,Reference Kashyap, Sharma and Sak5) . One recent clinical trial demonstrated significant improvement in anti-inflammatory markers when fisetin was supplemented to patients with colorectal cancer(Reference Farsad-Naeimi, Alizadeh and Esfahani6). Extensive pre-clinical studies have demonstrated the neuroprotective potential of fisetin associated with several neurological diseases, stroke and traumatic brain injury(Reference Pal, Diamond and Strickland2,Reference Zhang, Wang and Zhou7,Reference Currais, Farrokhi and Dargusch8) . A clinical study in stroke patients showed that fisetin was able to improve the treatment outcomes, particularly in individuals, with delayed onset-of-treatment(Reference Wang, Cao and Wu9). Emerging discoveries indicated the potential health benefits of fisetin in aging as a senolytic through the reduction of senescent markers and age-related pathologies(Reference Zhu, Doornebal and Pirtskhalava10,Reference Yousefzadeh, Zhu and McGowan11) . Pre-clinical studies have also indicated its potential for longevity extension(Reference Yousefzadeh, Zhu and McGowan11–Reference Grynkiewicz and Demchuk13). Many other pre-clinical studies associated with diabetes and related complications(Reference Prasath and Subramanian14,Reference Ge, Xu and Qin15) , obesity and comorbidities(Reference Jung, Kim and Ahn16–Reference Liou, Wei and Chen18), cardiovascular disease(Reference Ge, Xu and Qin19,Reference Hu, Feng and Dai20) and various inflammatory conditions(Reference Zheng, Feng and You21).

Fig. 1. Chemical structure of fisetin and its active metabolite, geraldol.
Previous in vivo studies have shown that fisetin is not generally detectable in systemic circulation because it rapidly undergoes metabolism to its glucuronide and sulphate conjugated forms(Reference Shia, Tsai and Kuo22). In addition, a biologically active metabolite was identified as 3, 4′, 7-trihydroxy-3′-methoxyflavone (geraldol; Fig. 1), which was shown to be a more potent cytotoxic compound against tumour cells than fisetin(Reference Touil, Auzeil and Boulinguez23). In vitro studies demonstrated that, like fisetin, geraldol could localise to the nucleoli in cultured cells, which is not the case with other structurally similar flavonols(Reference Krasieva, Ehren and O'Sullivan24).
A thorough review of the literature revealed that fisetin has not been reported to induce any toxicity upon in vitro, in vivo or in the limited clinical studies performed. In the only one available animal study by Currais et al. (Reference Currais, Prior and Dargusch25), no toxicity was reported up to 2000 mg/kg b. wt. of fisetin when administered orally as a single oral dose to mice. In the long-term (90 d) toxicity evaluation, supplementation of fisetin in the diet (0⋅05 % w/w) showed no toxic manifestations including organ toxicity. Furthermore, Ames test observed no mutagenicity(Reference Currais, Prior and Dargusch25).
Despite the numerous potential health-promoting activities, clinical applications with fisetin have been hampered by its low water solubility (10⋅4 μg/ml)(Reference Bothiraja, Yojana and Pawar26) and low bioavailability(Reference Seguin, Brullé and Boyer27). Various animal studies have demonstrated that fisetin undergoes fast metabolism, enzymatic degradation and P-glycoproteins (p-gp)-mediated efflux in the gastrointestinal tract upon oral administration(Reference Kadari, Gudem and Kulhari28). The serum levels of free fisetin declined within minutes following intravenous administration (t 1/2 = 2⋅7 min) and were only barely quantifiable in very limited samples up to 90 min following oral administration (t 1/2 not quantifiable)(Reference Shia, Tsai and Kuo22). These constraints associated with the bio accessibility of fisetin necessitate the development of new approaches to improve its bio-absorption as well as pharmacokinetic properties.
Numerous technologies and delivery systems based on nanoparticles, liposomes, phospholipid complexes, micelles and adjuvants have emerged to outwit the issue of poor bioavailability and absorption of flavonoids in the past decade(Reference Bothiraja, Yojana and Pawar26,Reference Seguin, Brullé and Boyer27,Reference Pooja, Babu and Kulhari29,Reference Singh, Rawat and Semalty30) . However, while most of these methods displayed improved bioavailability in intraperitoneal(Reference Seguin, Brullé and Boyer27) or intravenous(Reference Bothiraja, Yojana and Pawar26) administration, limited oral supplementation studies have been reported(Reference Kadari, Gudem and Kulhari28). We have recently developed a new technology, FENUMAT™, to improve the bioavailability of lipophilic bioactive’ using fenugreek soluble dietary fibre (galactomannan) as a physical hydrogel scaffold for the sustained delivery of self-emulsified colloidal forms, resulting in better absorption and stability in the intestinal tract(Reference Abhilash, Kumar and Deepti31,Reference Krishnakumar, Ravi and Kumar32) . Hydrogels, the three-dimensional framework of hydrophilic polymeric molecules with extensive water holding capacity, are recently gaining great interest in a variety of biomedical applications, including drug delivery(Reference Vigata, Meinert and Hutmacher33,Reference Shoukat, Buksh and Noreen34) .
We hypothesised that the FENUMAT™ formulation of fisetin would significantly enhance the solubility, stability and hence bioavailability of fisetin. A modified FENUMAT™ technology to engulf nanomicellar forms of fisetin within the fenugreek galactomannan (FG) hydrogel network was developed as Hybrid-FENUMAT™. The encapsulation of micelles in hydrogel network has already been reported as a method of choice to overcome the stability issues(Reference Qin, Xu and Zhou35,Reference Lu, Zhang and Yang36) . The present study was aimed at demonstrating the human pharmacokinetics of fisetin for the first time as well as characterising and estimating the bioavailability of the newly developed Hybrid-FENUMAT™-Fisetin formulation (FF-20) in comparison with the standard unformulated fisetin (UF) when orally supplemented in healthy human subjects. Experiments were performed to assess pharmacokinetic parameters such as plasma concentration over time following the oral administration of fisetin, maximum plasma concentration (C max), time taken to reach the maximum concentration in plasma (t max), time taken to reduce the plasma concentration to half of its maximum observed concentration (t 1/2), and hence the gastrointestinal absorption and stability of the absorbed fisetin in circulation. Particle size analysis, zeta potential, high-resolution electron microscopy, infrared spectroscopy, differential scanning calorimetry and powder x-ray diffraction (PXRD) were employed to characterise FF-20.
Experimental methods
Preparation and characterisation of FF-20
UF extracted from Rhus succedanea with 98⋅2 % purity and the granular powder form of FF-20 was produced in the certified good manufacturing practice (GMP)-plant of M/s Akay Natural Ingredients, Cochin, India. FF-20 was prepared by a proprietary process using sunflower lecithin, sunflower oil and fenugreek galactomannans, following a gel-phase thin-film dispersion of fisetin micelles into galactomannan hydrogel matrix and subsequent evaporation.
Stability, crystallinity, fisetin-galactomannan interactions and surface morphology were studied using differential scanning calorimeter (DSC), Powder X-ray diffraction (PXRD), Fourier-transform infrared spectroscopy (FTIR) and scanning electron microscopy (sem). PXRD analysis was carried out on a Bruker D8 Advance instrument: target Cu, kα – 1⋅54 Å, filter – Ni, voltage 40 kV, the time constant 5 min/s; scanning rate 1°/min (Bruker AXS GmbH, Karlsruhe, Germany). FTIR spectra were recorded in a Nicolet iS50 FTIR Spectrometer (Thermo Fisher Scientific, Massachusetts, USA) and sem analysis was performed on (ZEISS Sigma 500 VP, ZEISS microscopy, Oberkochen, Germany). Dynamic light scattering (DLS) (Horiba SZ-100 particle size analyser, Horiba India Private Limited, Bengaluru, India) was employed for the determination of particle size and zeta potential. The high-resolution transmission electron microscope (HR-TEM) (JEOL JEM-2100 LaB6, Jeol Co Limited, Japan) was used for the structural characterisation in the solution.
Fisetin content was measured using a validated high-performance liquid chromatography (HPLC) analysis. The Nexera UPLC (Shimadzu, Kyoto, Japan) instrument with SPD-10AVP Photo Diode Array detector was used for analysis. The chromatographic separation was achieved on a Phenomenex C18 (150 mm × 4⋅6 mm) column at 30 °C. The results were acquired and processed using Shimadzu LC-solution version 6.42 software for data acquisition and processing. Mobile phase consisted of (A): 0⋅1 % formic acid in water and (B): 0⋅1 % formic acid in methanol with a gradient flow of 1 ml/min was used for analysis and the detection was performed at 360 nm.
Study materials
Identical two-piece hard shell gelatin capsules of the study materials were provided by Akay Natural Ingredients, Cochin, India along with a detailed certificate of analysis and declaration indicating its suitability for human supplementation. Each capsule contained 500 ± 25 mg of UF or FF-20. Around 50 mg of microcrystalline cellulose was present in each capsule as a flow-improving excipient. The samples were stored in a cool dry area (24 ± 1 °C) protected from light and moisture. HPLC analysis indicated 19⋅2 % fisetin content in FF-20 capsules and 98⋅2 % content in UF capsules.
Pharmacokinetic study design
Twenty-one volunteers were initially screened to ensure eligibility based on the inclusion and exclusion criteria and fifteen were selected (N 15; twelve males and three females aged 22–55 years; BMI 18–25 kg/m2) for the study. A structured medical interview by a qualified physician and blood analysis (biochemical and hematological) were conducted to include in the study. Only participants who were willing to abstain from alcohol and food items rich in fisetin (including tea, wine, berry fruits, apple, grapes and cucumber) for 2 d prior to the study date were enrolled in the study. Females of childbearing age were only included if they had a negative pregnancy test and complied with approved birth control methods during the study. Exclusion criteria also included pregnant or lactating women, individuals with known hypersensitivity to the investigational products, individuals who participated in a clinical trial in the past month, and any individual unwilling or unable to comply with the study protocol. The demographic details of the participants and their blood routine analysis are given in Supplementary Table S1.
A single dose, randomised, comparative, double-blinded cross-over design (Fig. 2) was adopted to evaluate the difference in the bioavailability and pharmacokinetic parameters (C max, AUC, t max and t 1/2) of free (unconjugated) fisetin and geraldol following the oral administration of a formulated and UF (FF-20 and UF). Individuals were randomised using the computer-generated randomisation method (randomizer.org) and identified by a three-digit randomisation code. Participants reported to the study site by 8:00 h± 30 min in a fasted state (≥10 h) and were given an oral dose of 1000 mg of UF or FF-20 as two capsules along with 200 ± 10 ml of water. Blood samples (2 ml) were collected 5 min (t 0) prior to oral dosing and subsequently at 0⋅5, 1, 2, 3, 5, 8 and 12 h after oral ingestion of UF or FF-20, using an indwelling venous cannula. There was a washout period of 10 d between the treatments with UF or FF-20. Participants were provided with standardised south Indian meals, each containing on average 400–700 calories consisting of protein (27–30 %), fat (20–24 %) and carbohydrates (35–40 %). Breakfast (rice, vegetables and egg), lunch (rice, chicken/fish and vegetables), snack and dinner (wheat, vegetables or chicken/fish) were provided at 1, 4, 8 and 12 h after dosing, respectively. Water was provided ad libitum. Plasma was separated by centrifugation at 11 950 g for 10 min at 4°C and stored for a maximum of 2 d at −20°C for analysis.

Fig. 2. Schematic representation of the pharmacokinetic study protocol.
Ethical standards
The study was performed in strict accordance with the clinical research guidelines of Government of India, following the protocol which is approved by a registered ethical committee: CTRI/2020/07/026748, dated 23/07/2020. A written informed consent was acquired from all the study participants prior to the study and was in agreement with the standard operating procedure of the Helsinki and ICH-GCP (International Conference of Harmonization for Good Clinical Practice).
Triple Quadrupole QTRAP- UPLC-MS/MS analyses of fisetin and geraldol in plasma samples
Fisetin and geraldol in plasma samples were extracted and subjected to UPLC-ESI-MS/MS analyses as follows. Briefly, 1 ml of plasma was extracted with 4 × 1 ml of ice-cold acetonitrile and centrifuged at 4°C at 7000 rpm for 15 min. The supernatant was separated, filtered through a 0⋅45 μm syringe filter, and 3 μl was injected. Separation was achieved with Phenomenex Kinetex F5 column (150 × 4⋅6 mm, 2⋅6 μm), kept at 25°C. The mobile phase consisted of (A) 0⋅1 % formic acid in water and (B) 0⋅1 % formic acid in acetonitrile, set at a linear gradient of 50–100 % B within 7 min at 0⋅5 ml/min flow rate. A negative ion mode multiple reaction monitoring (MRM) was employed for ESI-MS/MS (4500 QTRAP, AB sciex, Singapore). MRM transitions for fisetin were m/z 284⋅8 → 135⋅1, 284⋅8 → 120⋅8 and that for geraldol were m/z 299⋅1 → 283⋅60, 299⋅1 → 148. Analyst software was employed for data acquisition. Range and linearity of the extraction efficiency were determined by spiking the internal standard, salbutamol (50 ng/ml), into plasma followed by LC-MS/MS analysis. When the blank plasma samples and fisetin/geraldol spiked plasma samples were extracted and analysed, they were found to be free from any interference at the respective retention times of each of the analytes. Matrix matched calibration curves for fisetin from plasma were generated by plotting the concentrations of fisetin and geraldol against peak response and were found to be linear over the concentration range of 1–1000 ng/ml, with an R 2 value of 0⋅9976 and 0⋅9961, respectively. Extraction efficiency of fisetin from plasma was found to be greater than 84 % and that for geraldol was 81 %, as confirmed by the internal standard, salbutamol. The accuracy and precision of the method were within the acceptable limits of 20 % as specified in ICH guidelines.
Statistical analysis
Statistical analysis was performed using SPSS version 27. All data were expressed as mean ± sd. The pharmacokinetic parameters C max, t max, t 1/2 and AUC and their mean and percentage changes from the baseline were performed using Analysis of Variance (ANOVA) followed by Dunnett's test to estimate the differences between the groups. P < 0⋅05 were considered as statistically significant. The pharmacokinetic parameters were deduced from the plasma concentration v. time plot using GraphPad Prism 9.2.0.
Results
Characterisation of FF-20
The FTIR analysis of UF showed characteristic bands at 3384 and 3254 cm−1 corresponding to O–H stretching. The aromatic C=C vibration was observed at 1510 cm−1 and the bands at 1087 and 1194 cm−1 were attributed to the C–O–C group vibrations. The in-plane C–H bending, C–H wagging and O–H bending vibrations of the ring (i) & (ii) (inset in Fig. 3(a)) were observed in the wavelength region 1500–1300 cm−1(Reference Ghosh, Singha and Chaudhury37). The C–OH stretching vibrations of the ring (ii) were observed below 1300 cm−1. In the formulated fisetin (FF-20), all the earlier-mentioned peaks were present along with some additional peak's characteristic of lecithin (1025 cm−1) and liposomes (2955 and 2850 cm−1), indicating the absence of any chemical modifications to fisetin. The characteristic peaks of FG were also evident at 2923 and 1055 cm−1, indicating the presence of FG, UF and lecithin in FF-20 (Fig. 3(a))(Reference Rashid, Hussain and Ahmed38).
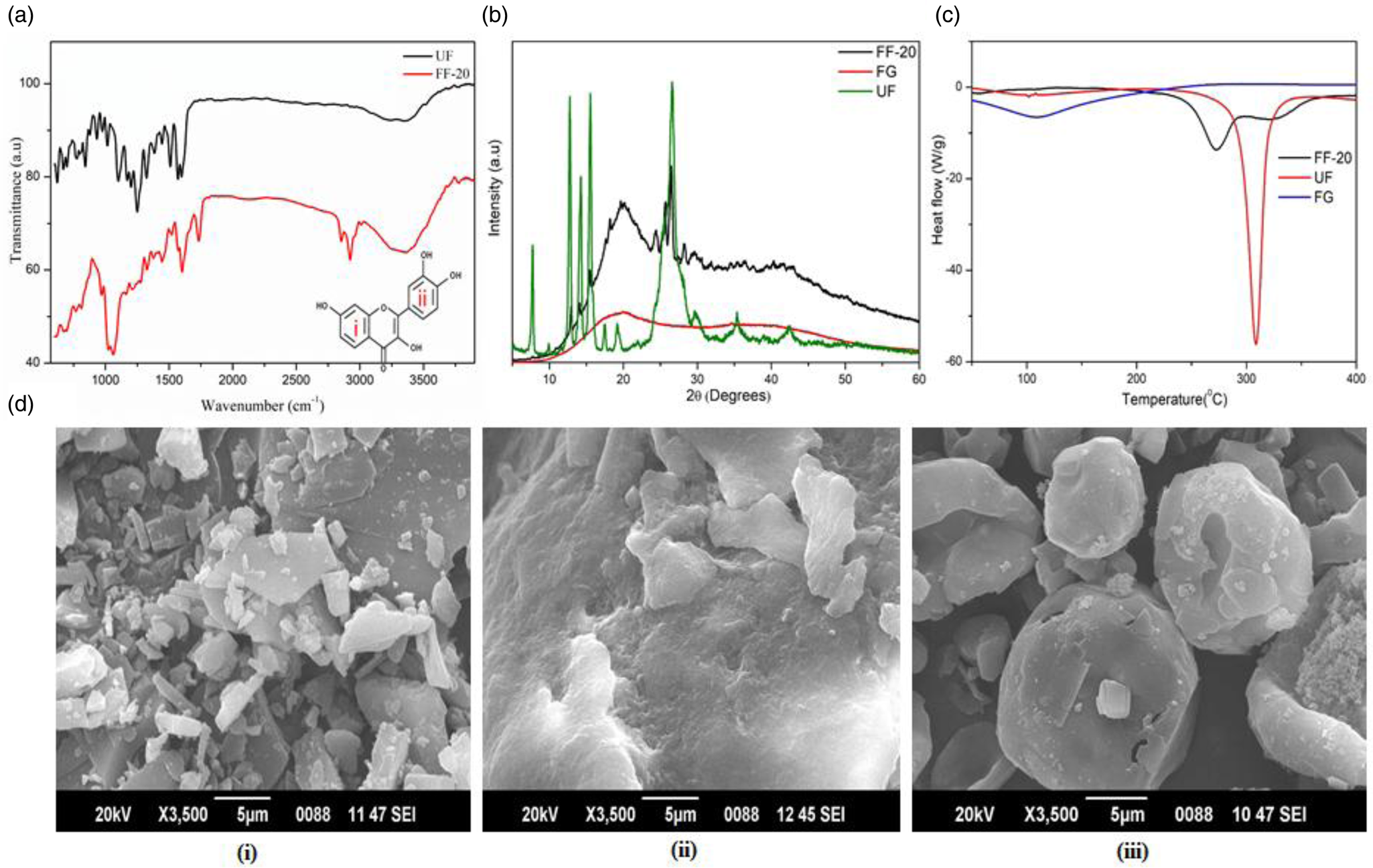
Fig. 3. (a) FTIR spectra of FF-20 and UF, (b) powder XRD diffractogram of UF, FG and FF-20, (c) differential scanning calorimetry of UF, FG and FF-20, (d) sem images of (i) UF, (ii) FG and (iii) FF-20.
Fig. 3(b) shows PXRD pattern of FG, UF and FF-20. FG provided a typical flat diffractogram corresponding to the amorphous nature of the powder. UF on the other hand provided a pattern indicating a significant crystallinity, as evident from the multiple sharp peaks. In the case of FF-20, the number of peaks and their intensity were found to be significantly reduced as compared to UF. The characteristic amorphous pattern of the galactomannan fibre was also evident in FF-20 diffractogram, indicating the possible encapsulation of crystalline fisetin in the amorphous matrix of galactomannan fibre network.
DSC analysis showed a sharp endothermic peak at 310°C corresponding to the melting of UF used for the formulation. Galactomannan fibre on the other hand had no characteristic peak but exhibited a valley like plot. However, FF-20 exhibited a broad less intense endothermic shift in the region 270–320°C with a less intense peak at 274°C, which may be attributed to the depression in the melting point of fisetin due to its extensive encapsulation effect in the fibre matrix (Fig. 3(c)).
The sem image of UF, FG and FF-20 are shown in Fig. 3(d) (i, ii, iii), respectively. The crystalline nature of fisetin was clear from Fig. 3(d)(i). The galactomannan fibre, on the other hand, was highly amorphous with no definite structure [Fig. 3(d)(ii)]. FF-20 showed mainly the spherical form with a smooth, translucent surface, indicating the homogeneous encapsulation of fisetin into the galactomannan hydrogel matrix [Fig. 3(d)(iii)]. It was further observed from encapsulation efficacy determination that fisetin was encapsulated with a high encapsulation efficiency of 93⋅3 ± 0⋅78 % as determined by HPLC.
Particle size analysis of the initial micellar preparation of fisetin before impregnating into the hydrogel matrix by DLS showed uniformity and stability with an average particle size of 50 ± 15 nm (Fig. 4(a)). FF-20 solution prepared by ultra-sonication and centrifugation on the other hand showed particles of about 151⋅6 ± 5⋅1 nm, indicating that the effective increase in the hydrodynamic volume resulted from the surface modification by the hydrophilic galactomannan chains (Fig. 4(b)). Upon TEM analysis of this solution, a chain-like structure was observed with relatively dark spots in various areas (Fig. 4(c)). The size of these dark spots was also found to be in the range of 50 nm, possibly due to the higher concentration of the micelles in the galactomannan network as a micellar/hydrogel composite.

Fig. 4. DLS and TEM analysis of FF-20 in solution: (a) hydrodynamic size distribution of fisetin micelle leached from FF-20 granular powder during in vitro dissolution after 1 h at pH 7⋅0 (possible micellar structure of fisetin in solution is depicted in the inset); (b) hydrodynamic size distribution of FF-20 solution obtained after ultrasound-aided dissolution in water at pH 7⋅0 (possible galactomannan-bound micellar structure of fisetin in solution is depicted in the inset); (c) TEM image of FF-20.
Pharmacokinetics of fisetin
Due to the low bioavailability, detection of the UF in individuals was only quantifiable when supplemented at 1000 mg dose, which was equivalent to approximately five times more fisetin content than FF-20 (1000 mg of FF-20 contained only 192 mg fisetin). All results shown were adjusted for the difference in fisetin content. The time course for the plasma fisetin concentration, shown in Fig. 5(a), was significantly higher (****P < 0⋅0001) at all the time points detected (0⋅5, 1, 2, 3, 5, 8 and 12 h post-supplementation) when individuals were administered with FF-20. Fisetin levels were quantifiable up to 8 h after dosing with FF-20 compared to only 2 h after dosing with UF. The average plasma concentration of fisetin (Fig. 5(a) inset) determined in the individuals when supplemented with FF-20 (AUC0–12 h = 341⋅4 ng*h/ml) was 26⋅9-fold greater than when supplemented with UF (AUC0–12 h = 12⋅67 ng*h/ml).

Fig. 5. (a) Time course of fisetin plasma concentration with area under the curve, AUC (inset) and (b) time course of geraldol plasma concentration with area under the curve, AUC (inset), both after supplementation with FF-20 and UF; N = 15. Statistical analysis was performed using SPSS software version 27 and all data points were expressed as mean ± sd. P < 0⋅05 was considered statistically significant. *P < 0⋅05, ***P < 0⋅001, ****P < 0⋅0001; GraphPad Prism Version 9.2.0 was used to plot the graph.
The C max for fisetin was 238⋅2 ng/ml at a t max of 1⋅24 h for FF-20 and that was only 9⋅97 ng/ml with a t max of 0⋅88 h for UF. The t 1/2 was extended to 1⋅51 h when supplemented with FF-20 compared to the t 1/2 of 1⋅14 h for UF (Table 1).
Table 1. Pharmacokinetic parameters for fisetin and geraldol: C max, t max, t 1/2 and AUC values, which were normalised to adjust for the higher fisetin intake in the unformulated fisetin (UF)

UF, Unformulated fisetin; FF-20, Hybrid-FENUMAT-fisetin formulation; C max, Maximum plasma concentration; t max, Time taken to reach the maximum concentration in plasma; t 1/2, Time taken to reduce the plasma concentration to half of its maximum observed concentration; AUC, Area under the curve.
Mean values were significantly different from those of the UF: *P < 0⋅05, ***P < 0⋅001, ****P < 0⋅0001.
Pharmacokinetics of geraldol
In addition to evaluating the time course for fisetin in the plasma, geraldol which is an active methoxylated metabolite of fisetin was also analysed to understand its conversion in human subjects. The time course for the geraldol plasma concentration, shown in Fig. 5(b), was also significantly higher (****P < 0⋅0001) at all the time points detected (0⋅5, 1, 2, 3, 5, 8 and 12 h post fisetin supplementation) when individuals were provided with FF-20 compared to UF. The plasma concentration of geraldol was quantifiable only up to 2 h after dosing with UF. The average plasma concentration of geraldol (Fig. 5(b) inset) in the individuals when supplemented with FF-20 (AUC0–12 h = 227⋅14 ng*h/ml) was 11⋅1-fold greater than when supplemented with UF (AUC0–12 h = 20⋅48 ng*h/ml). Additionally, the AUC ratio of geraldol to fisetin after supplementation with FF-20 was 0⋅67, which was more than two times lower than after supplementation with UF (1⋅62) (Table 1).
The C max, t max and t 1/2 of geraldol also followed a similar pattern to fisetin (Table 1). The C max for geraldol occurred at 0⋅9 h after dosing with UF and was shifted to 1⋅14 h for FF-20. The C max for FF-20 was 239⋅2 ng/ml compared to the UF plasma samples (24⋅0 ng/ml). The C max ratio of geraldol to fisetin was 2⋅4-fold higher in the UF plasma samples but was about the same in the FF-20 plasma samples. The t 1/2 for UF was also slightly extended to 1⋅59 h in individuals when supplemented with FF-20 compared to 1⋅18 h for UF.
Adverse events/safety
The study did not show any significant adverse events or clinical signs due to fisetin supplementation. However, two cases of gastrointestinal issue were reported, one was a bloating sensation and the other one was a decrease in appetite. These observations were same upon both UF and FF-20 intake.
Discussion
The present study was aimed at the characterisation and investigation of human pharmacokinetics of a naturally existing fisetin isolated from a plant source by solvent extraction (UF) and a self-emulsifying water-dispersible formulation of fisetin using the green technology known as Hybrid-FENUMAT™ (FF-20). Fisetin, despite showing many potential health benefits, has low bioavailability, mainly owing to its high lipophilicity (log P: 3⋅2), poor water solubility (10 μg/ml) and rapid metabolism in pre-clinical studies(Reference Mehta, Pawar and Mahadik3). So, a high and frequent dosage is often speculated for an optimum therapeutic efficacy, and this may lead to adverse effects. The clinical utility and functional usage of fisetin are thus limited and improvements in the bioavailability may result in the enhancement of efficacy and dosage reduction. Oral delivery forms are typically preferred over infusions and there is a need for natural, food-grade delivery forms for alternative applications of nutritionally relevant molecules in nutraceuticals and functional foods.
Though various attempts, including liposomal, nanoemulsion, nanomicelles and solid-lipid nanoparticle formulations have been reported to improve the bioavailability of phytonutrients, many of such formulations face various challenges. These include degradation under harsh stomach conditions, low loading levels of bioactives, liquid state and hence poor storage stability due to high free energy and rapid agglomeration, usage of high levels of synthetic emulsifiers, high cost, and regulatory issues due to the nano size(Reference Lu, Zhang and Yang36,Reference Sercombe, Veerati and Moheimani39) . In the case of fisetin, most of the in vivo bioavailability studies delivered fisetin intravenously or intraperitoneally with limited studies focused on bioavailability(Reference Mehta, Pawar and Mahadik3). One study using a nanoparticle formulation, encapsulated fisetin in poly-lactide-co-glycolic acid (PLGA), resulted in an increased bioavailability in mice(Reference Kadari, Gudem and Kulhari28), indicating the feasibility of enhancing fisetin bioavailability by suitable encapsulation techniques with particle size reduction, stability and increased solubility.
To the best of our knowledge, no human pharmacokinetics studies have been reported for fisetin. The present results indicated that poor bioavailability is also a significant issue in human subjects, as previously reported in animal studies. Detectable fisetin levels were very low and not quantifiable by UPLC-ESI-MS/MS methods when individuals were supplemented with less than 1000 mg of UF, though it was detected at the 1 h time point upon dosing of 500 mg (data not shown). Additionally, fisetin levels could only be quantified in the plasma up to 2 h after the individuals were supplemented with 1000 mg UF. This may be a result of rapid metabolism as previously described in pre-clinical studies(Reference Shia, Tsai and Kuo22). To further evaluate this, we estimated the presence of geraldol, an active metabolite of fisetin in the plasma samples. The geraldol C max and AUC0–12 h levels were 2⋅4-fold and 1⋅6-fold higher than fisetin, respectively when supplemented with UF. Although geraldol is only one of the fisetin metabolites, the data support that the rapid conversion of fisetin to geradol also occurs in the human subjects, however further analysis of other metabolites would be beneficial in future studies to better understand its metabolism.
Previous studies have shown improved pharmacokinetic properties for the natural formulations of lipophilic phytonutrients, either alone (curcumin, capsaicin) or a co-delivery (curcumin and boswellic acid), based on FG dietary fibre as a hydrogel matrix, FENUMAT™(Reference Abhilash, Kumar and Deepti31,Reference Krishnakumar, Ravi and Kumar32,Reference Joseph, Abhilash and Johannah40) . In the present study, a modified version of FG delivery technology was developed as Hybrid-FENUMAT™ to improve the delivery and bioavailability of fisetin. It was prepared as a novel micelle/hydrogel composite by the uniform incorporation of lecithin-based micelles of fisetin into the hydrated pockets created by the conformationally restricted FG molecular network. FTIR analysis of FG, UF and FF-20 demonstrated the composite structure and mode of interactions between the individual components. The formulation, FF-20 was obtained as a granular free-flowing powder with a particle size distribution in the range of 150–400 μm. The amorphous nature of FG and the crystalline nature of UF were clear from their PXRD diffractograms. In the case of FF-20, the characteristic nature of both FG and UF was visible, with broad and less intense peaks corresponding to fisetin. The number of peaks was also reduced as compared to UF, indicating the significant amorphous nature of FF-20 due to the encapsulation of crystalline fisetin into the amorphous FG matrix. The encapsulation of fisetin in FG matrix in FF-20 was further clear from the DSC analysis, which showed a broad and less intense endothermic band at 270–320°C region, rather than the sharp endothermic peak at 310°C due to the melting of UF. Further sem analysis confirmed that the gel-phase thin film evaporation process employed in the present formulation of FF-20 was successful in the homogeneous encapsulation of fisetin micelles into the amorphous galactomannan hydrogel matrix to form the spherical, amorphous micelle/hydrogel composite particles of FF-20.
FF-20 exhibited enhanced solubility compared to the UF. This is due to the uniform anchoring of the stable micelles of fisetin in the hydrogel matrix of galactomannan to form the micelle/hydrogel composite. When in contact with water or gastrointestinal fluid, FF-20 swells and the stable micellar particles of fisetin leach out, since it is a soft physical hydrogel with no chemical crosslinking. This is clear from the particle size analysis of the solution of FF-20 under intestinal pH conditions of 6⋅8 ± 0⋅2 which showed colloidal particles of about 151⋅6 ± 5⋅1 nm (zeta potential of −21⋅3 mV), indicating an in vivo nano delivery of fisetin. The relative enhancement in the hydrodynamic size of the dissolved particles from the size of the micelles is due to the surface modification by the hydrophilic galactomannan network, as shown in the inset of Fig. 4(b). The surface exposed hydroxyl groups (−OH) of the galactomannan can establish effective hydrated volume by establishing intermolecular H-bonding with the surrounding water molecules. The strong interaction and entrapment of nanomicelles in the hydrogel matrix was further evident from the TEM studies, which showed a chain-like structure with relatively dark spots of around 50 nm size in various areas (Fig. 4(c)). The dark spots may be attributed to the higher concentration of the micellar in the galactomannan network. Thus, it can be concluded that the nanomicellar structures of fisetin are uniformly engulfed into the extensively hydrated galactomannan network to form a stable self-emulsified colloidal solution with enhanced solubility, stability and hence better absorption. So, the formation of FF-20 from UF, lecithin and galactomannan as a hybrid-hydrogel (micelle/hydrogel), and its in vivo delivery mechanism can be considered as a natural self-emulsifying reversible hybrid-hydrogel system (N-SERH), schematically depicted as Fig. 6.

Fig. 6. (a) Schematic representation of the formulation of FF-20 and (b) schematic representation of the in vivo release of fisetin from hydrogel matrix.
Surface modification and functionalisation of nano structures like liposomes and micelles by hydrophilic polymers such as polyethylene glycol (PEG) or by the entrapment into the stable hydrophilic polymeric networks, such as hydrogels, is an emerging area of drug delivery and biomedical engineering(Reference Vieira and Gamarra41,Reference Cabral, Miyata and Osada42) . The approach has been shown to improve the pharmacokinetics, blood–brain barrier permeability and tissue distribution, by protecting the vehicle from opsonisation, minimising the binding with plasma proteins and by reducing the rate of clearance from the systemic circulation(Reference Vieira and Gamarra41). The surface coating with the galactomannan chains in the present formulation of FF-20 thus provides a ‘capping effect’ to protect the fisetin micelles from the harsh stomach conditions as well as from the pre-systemic biotransformations catalysed by the phase I metabolising enzymes. The mucoadhesive character of the galactomannan-bound nanomicellar particles also helps the strong binding within the microvilli and hence to attain high local concentration to facilitate the diffusion-mediated transport process.
The current pharmacokinetic study in healthy individuals showed improved bioavailability with the oral delivery of this novel encapsulated form of fisetin, as indicated by the improved absorption (23⋅9-fold increase in C max), bioavailability (26⋅9-fold enhancement in AUC0–12 h), enhanced t 1/2 and t max for FF-20, which contained only 192 mg of fisetin as compared to 982 mg in UF. While no fisetin was observed after 2 h of consumption of 982 mg fisetin from UF, plasma level was quantifiable up to 8 h after the individuals consumed FF-20, indicating that the hydrogel formulation provided protection of the fisetin for a sustained delivery. Furthermore, the conversion of geraldol may have been reduced and/or delayed with the encapsulated formulation which was indicated by the lower geraldol to fisetin ratio as seen with the C max (equal plasma concentrations) and AUC0–12 h (0⋅67-fold lower). Overall, the pharmacokinetic data support the improved delivery and bioavailability of fisetin in healthy volunteers upon oral consumption of this novel, food grade and hybrid-hydrogel formulation.
Conclusion
In conclusion, the present study demonstrated the poor bioavailability of fisetin in human subjects, as previously observed with animal studies. Furthermore, an innovative formulation was developed and shown to significantly improve the bioavailability of fisetin when administered orally in a human clinical study. FF-20 is a novel formulation of fisetin using the green Hybrid-FENUMAT™ technology to engulf lecithin-based micelles of fisetin within the three-dimensional pockets of FG hydrogel network to form natural hybrid-hydrogel (micelle/hydrogel composite) system. FF-20 is produced in powder form, suitable for extensive swelling under gastrointestinal conditions to leach mucoadhesive galactomannan coated nanomicellar forms of fisetin. The encapsulation thus helps to protect fisetin from environmental factors such as low stomach pH conditions and extensive intestinal biotransformation, and to offer a sustained intestinal delivery of self-emulsified nanomicelle for better absorption. This is evident from the significantly higher bioavailability and pharmacokinetic properties of free (unconjugated) fisetin and geraldol in healthy volunteers. The lack of measurements of fisetin glucuronides and sulphates may be a limitation of the present study, which would be a direction for future studies. With no adverse events reported from this study and previous studies, this natural self-emulsifying reversible hybrid-hydrogel formulation provides a safe and viable delivery system for fisetin in order to further explore and verify its potential health benefits.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/jns.2022.72.
Acknowledgments
The authors thank Dr Prasanth Shanmughan at R&D of Akay Natural Ingredients for the characterisation of FF-20.
K. I. M. is the principal investigator who developed the hybrid-hydrogel FENUMAT technology, supervised the study and reviewed the manuscript. A. J. conducted the formulation development trials. A. B. validated the tandem mass spectrometry method for the analysis of the biomatrix samples and conducted the pharmacokinetic study. B. M. arranged funding and reviewed the manuscript. A. J. C. analysed data, wrote and reviewed the manuscript. A. G. S. conceived the idea to apply the technology to fisetin and reviewed the manuscript.
The present study was financially supported by Akay Natural Ingredients, Cochin, India.
FENUMAT™ and Hybrid-FENUMAT™, the technologies used in the present formulation of fisetin are patented and registered by Akay Natural Ingredients, Cochin, India. Life Extension is a nutraceutical company based in USA who sell the fisetin formulation (FF-20) under the name Bio-Fisetin.