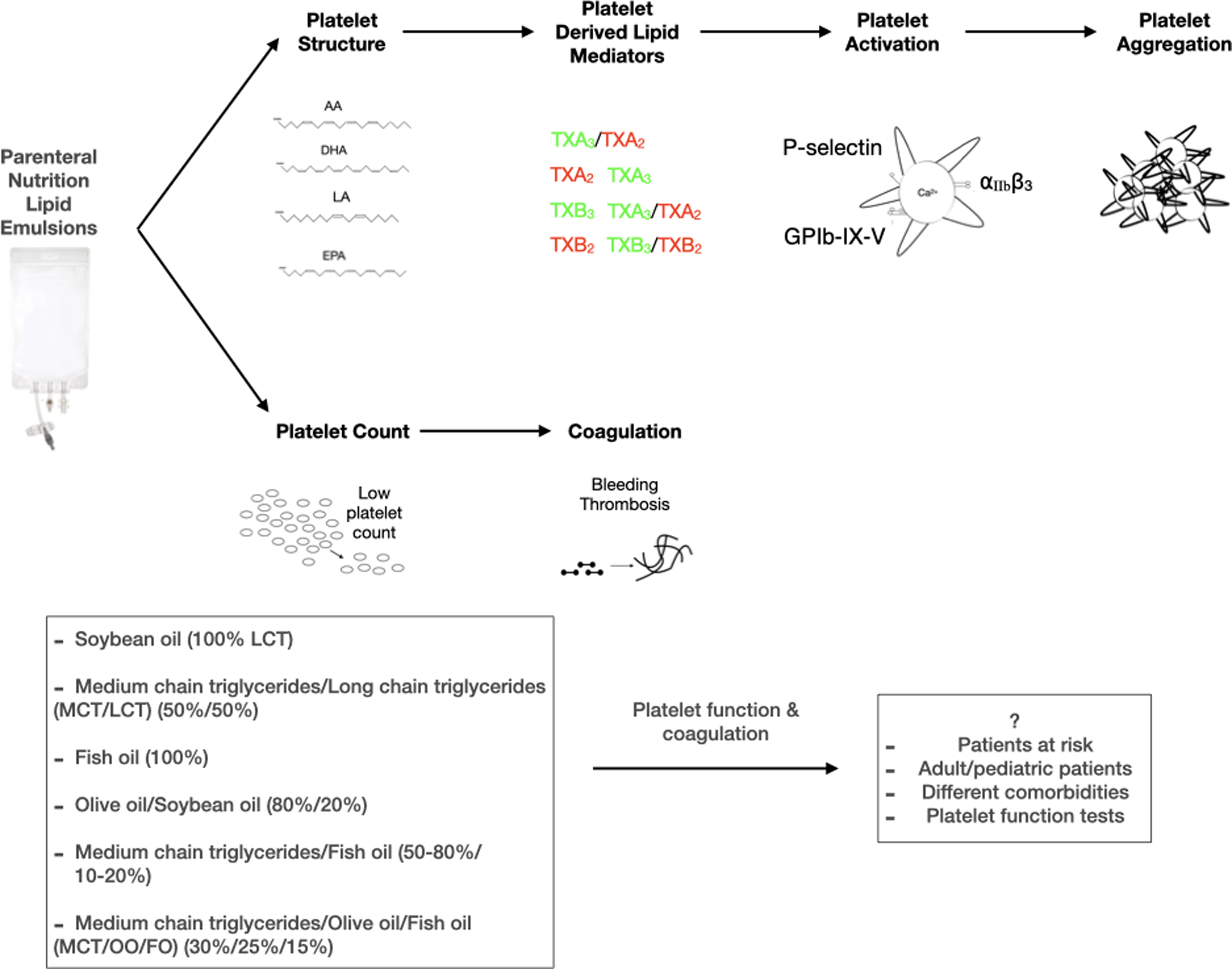
Introduction
Lipid emulsions are vital components of parenteral nutrition (PN) solutions(Reference Kolaric, Pukšič and Goričanec1–Reference Wanten and Calder3) and provide about 25-40% PN non-protein energy(Reference Adolph, Heller, Koch, Koletzko, Kreymann, Krohn, Pscheidl and Senkal4,Reference Lapillonne, Fidler Mis, Goulet, van den Akker, Wu and Koletzko5) , prevent essential fatty acid deficiency(Reference Adolph, Heller, Koch, Koletzko, Kreymann, Krohn, Pscheidl and Senkal4,Reference Fell, Nandivada, Gura and Puder6,Reference Calder, Jensen, Koletzko, Singer and Wanten7) and may have anti-inflammatory, or immune-modulating properties(Reference Martindale, Berlana, Boullata, Cai, Calder, Deshpande, Evans, Garcia-de-Lorenzo, Goulet, Li and Mayer8,Reference Boullata, Berlana, Pietka, Klek and Martindale9) . The triglyceride (TG) component of lipid emulsions may be from different sources, including soybean oil (SO), olive oil (OO), safflower oil, coconut oil, and fish oil (FO)(Reference Adolph, Heller, Koch, Koletzko, Kreymann, Krohn, Pscheidl and Senkal4,Reference Raman, Almutairdi, Mulesa, Alberda, Beattie and Gramlich10) . First (SO), second (SO + medium-chain triglycerides)/third (OO), and fourth generation (FO) lipid emulsions can be identified as pro-inflammatory, inflammatory neutral, and anti-inflammatory, respectively(Reference Adolph, Heller, Koch, Koletzko, Kreymann, Krohn, Pscheidl and Senkal4,Reference Raman, Almutairdi, Mulesa, Alberda, Beattie and Gramlich10) .
Commercially available lipid emulsions contain primarily long-chain triglycerides (LCT) along with mixes, including medium-chain triglycerides (MCT), which are referred to as MCT/LCT(Reference Lapillonne, Fidler Mis, Goulet, van den Akker, Wu and Koletzko5). Soybean oil-based emulsions contain 44-62% of linoleic acid (LA) (Table 1)(Reference Mahan and Escott-Stump2,Reference Mundi, Salonen, Bonnes and Hurt11) and may have pro-inflammatory effects via eicosanoids synthesized from n-6 PUFA arachidonic acid (AA)(Reference Mundi, Salonen, Bonnes and Hurt11). Olive oil is reported to be more advantageous than SO because of its higher MUFA and lower n-6 PUFA content (Table 1)(Reference Mundi, Salonen, Bonnes and Hurt11). Similarly, FO lipid emulsions are used for their proposed anti-inflammatory and immunomodulatory effects(Reference Mahan and Escott-Stump2,Reference Wanten and Calder3,Reference Fell, Nandivada, Gura and Puder6,Reference Calder, Jensen, Koletzko, Singer and Wanten7,Reference Calder12) . These lipid emulsions may be used in combination with other lipid sources (SO, MCT, OO, and FO-containing lipid emulsions) to lower the amount of SO. Furthermore, they contain higher n-3 PUFA, α-tocopherol, and low amounts of plant phytosterols than SO-based lipid emulsions (Table 1)(Reference Mundi, Salonen, Bonnes and Hurt11).
Table 1. Comparison of lipid emulsions in lipid source and fatty acid composition

SO, soybean oil; MCT, medium-chain triglycerides; LCT, long-chain triglycerides; FO, fish oil; OO, olive oil; EPA, eicosapentaenoic acid; DPA, docosapentaenoic acid; DHA, docosahexaenoic acid; MUFA, monounsaturated fatty acids; PUFA, polyunsaturated fatty acids.
–Not detected or not reported (in either situation, there is little or none present).
a Data provided is from the manufacturer data sheets or(Reference Calder, Jensen, Koletzko, Singer and Wanten7, Reference Calder12).
b MCT lipid sources may be coconut, palm kernel or other tropical nut oils.
Parenteral nutrition may influence haemorheological parameters and platelet function(Reference Compagnoni, Schulzki, Thoeny and Reinhart13), and it is now well established that these complications may result from the lipid source of PN solutions. Lipid emulsions will likely affect platelet function due to the many biological functions (inflammatory and immune responses, coagulation, cell signalling)(Reference Mahan and Escott-Stump2,Reference Kelly, Bremner, Hartley and Flynn14–Reference Mehanna, Moledina and Travis16) . Platelets, the most abundant cells in the blood, are the vital cells responsible for haemostasis(Reference Versteeg, Heemskerk, Levi and Reitsma17,Reference Vasina, WM Heemskerk, Weber and R Koenen18) . The functional changes of platelets (adhesion, activation, spreading, secretion, aggregation, pro-coagulant activity, microparticle formation, clot retraction) followed by tissue damage or other pathophysiological conditions (atherosclerotic lesion, inflammation) exist to maintain haemostasis(Reference Harrison19,Reference Paniccia, Priora, Liotta and Abbate20) . Impaired platelet function and abnormal platelet number may result in bleeding or thrombus formation(Reference Harrison19,Reference Paniccia, Priora, Liotta and Abbate20) . A group of agonists activates platelets in physiological haemostasis and pathological bleeding or thrombosis, and a cascade of reactions occurs. It is important to note that increased responsiveness of platelets to agonists in conditions like endothelial damage or pro-inflammatory state over-activates platelets in favour of a thrombotic state(Reference Siljander, Munnix, Smethurst, Deckmyn, Lindhout, Ouwehand, Farndale and Heemskerk21) (Fig. 1).

Fig. 1. a. Signalling mechanisms during platelet activation. A resting platelet owns a discoid shape with α and δ granules, and an activated platelet becomes round and forms pseudopods. Strongly activated platelets have high cytosolic Ca2+. Collagen is exposed from the damaged endothelium and binds to GPVI and GPIb-IX-V with another ligand vWF. Fibrinogen activates the platelet through the integrin αIIbβ3 (GPIIb/IIIa). Integrin activation leads to platelet aggregation. Agonists ADP and thrombin take roles through receptors P2Y1, P2Y12, and PAR. Activated platelets express PS on their surface. An adhesive molecule, P-selectin, is expressed on an activated platelet. b. Scheme of the coagulation cascade. Steps into the coagulation cascade: fibrinogen produces fibrin through thrombin. Fibrin is responsible for forming a tight thrombus. c. Scheme of the produced lipid mediators via the platelet plasma membrane. Lipid mediators form through the release of platelet membrane phospholipid fatty acids, which may affect platelet activation/aggregation. 12-HETE may increase or decrease platelet activation. TXA2 and TXB2 are pro-coagulants and increase platelet activation. TXA3 reduced platelet activation, whereas endothelial cell-derived PGI2 inhibits platelet activation. DHA is known to inhibit AA formation in human platelets. AA, arachidonic acid; ADP, adenosine diphosphate; COX, cyclooxygenase; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; LOX: lipoxygenase; PG, prostaglandin; PS, phosphatidylserine; TX, thromboxane; vWF, von Willebrand factor; 12-HETE, 12-hydroxyeicosatetraenoic acid.
Platelet function tests to monitor the risk of bleeding or predict thrombosis are essential in the hospital(Reference Harrison19,Reference Paniccia, Priora, Liotta and Abbate20) . Traditional tests such as bleeding time and aggregometry are widely used(Reference Harrison19,Reference Paniccia, Priora, Liotta and Abbate20) . Partial thromboplastin time (PTT) and prothrombin time (PT) are basic clotting tests used to exclude coagulation defects(Reference Harrison19,Reference Paniccia, Priora, Liotta and Abbate20,Reference Hoffman and Monroe22) . As the gold standard of platelet function(Reference Paniccia, Priora, Liotta and Abbate20) aggregometry tests that give responses to a panel of agonists(Reference Harrison19,Reference Paniccia, Priora, Liotta and Abbate20) are still used; however, these tests are proposed not to define responses to weak agonists on a clinical basis(Reference Harrison19,Reference Paniccia, Priora, Liotta and Abbate20) . Platelet function tests such as flow cytometry can explore the assessment of signalling processes and activation properties(Reference Harrison19,Reference Paniccia, Priora, Liotta and Abbate20,Reference Cauwenberghs, van Pampus, Curvers, Akkerman and Heemskerk23) . Flow cytometry and cell morphology under the microscope can measure platelet activation, such as integrin activation, secretion of granule contents, and phosphatidylserine (PS) exposure(Reference Cauwenberghs, van Pampus, Curvers, Akkerman and Heemskerk23). Platelet glycoproteins, activation markers, and platelet function are investigated(Reference Harrison19,Reference Paniccia, Priora, Liotta and Abbate20) . Moreover, studies also investigate the phospholipid composition of platelet plasma membranes that is tightly regulated for haemostasis(Reference Heemskerk, Bevers and Lindhout24) and can also signify platelet activation; for example, PS is exposed highly on the surface before coagulation(Reference Heemskerk, Bevers and Lindhout24) (Fig. 1).
Thrombocytopenia, platelet dysfunction, hypercoagulation, and bleeding were reported in patients when lipid emulsions were introduced in PN solutions. Thromboembolic events, including thrombosis, thromboembolism(Reference Kakzanov, Monagle and Chan25–Reference Harvey, Xu, Pavlina, Zaloga and Siddiqui29), major bleeding(Reference Ball and Allington30), vena cava syndrome, thrombocytopenia, and heparin complications(Reference Barco, Heuschen, Salman, Brekelmans, Serlie, Middeldorp and Coppens31), are seen in many patients due to PN. During hospital stays, patients are prone to thromboembolic events due to reduced physical activity(Reference Stoetzer, Nickel, Weißig, Großheim, Scheinichen, Doll and Jüttner32). PN might affect haemostasis and could potentially increase the risk of bleeding or postoperative thrombosis. Also, PN is used in patients (critically ill, patients on chemotherapy, bone marrow transplantation) who are likely to develop thrombocytopenia(Reference Ball and Allington30). Intravenous (IV) fat emulsion infusions have been associated with bleeding complications, thrombocytopenia, effects on cholesterol, triglyceride, glucose, insulin and blood pressure(Reference Beaulieu, Vitseva, Tanriverdi, Kucukural, Mick, Hamburg, Vita and Freedman33).
On the other hand, lipid emulsion infusion may be used for the benefit of patients to prevent preoperative infarction, postoperative occlusion of coronary arteries(Reference Veljović, Mihajlović, Subota, Antunović, Jevdjić, Udovicić, Popadić and Vulović34), and severe hypercoagulability cases(Reference Kapp, Duckert and Hartmann35). Potentially, some lipid emulsions may have anti-atherogenic properties(Reference Hayek, Fuhrman, Levy, Aviram and Brook36,Reference Aviram and Deckelbaum37) , and these effects should be defined to be used in patients with accelerated atherosclerosis as the inhibition of platelet aggregation could lead to arrest or regression of atherosclerotic process(Reference Hayek, Fuhrman, Levy, Aviram and Brook36).
The influence of PN lipid emulsions on platelet function and coagulation is a controversial issue(Reference Stoetzer, Nickel, Weißig, Großheim, Scheinichen, Doll and Jüttner32). Although ESPEN defines lipid emulsions as generally safe concerning platelet activity(Reference Calder, Adolph, Deutz, Grau, Innes, Klek, Lev, Mayer, Michael-Titus, Pradelli and Puder38,Reference Singer, Blaser, Berger, Alhazzani, Calder, Casaer, Hiesmayr, Mayer, Montejo, Pichard and Preiser39) , there needs to be more knowledge on the effects of PN solution contents on normal platelet functions, as well as in bleeding and thrombotic disease processes. Platelet activation plays a central role in the pathogenesis of thromboembolic incidents(Reference Stoetzer, Nickel, Weißig, Großheim, Scheinichen, Doll and Jüttner32), and platelet dysfunction is a significant cause of excessive bleeding(Reference Veljović, Mihajlović, Subota, Antunović, Jevdjić, Udovicić, Popadić and Vulović34). Therefore, the PN treatment should not lead to platelet aggregation, which might lead to atherosclerotic lesions and acute thrombosis(Reference Stoetzer, Nickel, Weißig, Großheim, Scheinichen, Doll and Jüttner32), or platelet dysfunction and bleeding(Reference Veljović, Mihajlović, Subota, Antunović, Jevdjić, Udovicić, Popadić and Vulović34). In this review, we gathered and summarized studies investigating parenteral nutrition lipid emulsions and their effects on platelets in order to provide clinically-relevant updates on parenteral nutrition and haemostasis.
Methods
This review gathered human studies investigating parenteral nutrition lipid emulsions and their effects on platelets. PubMed, Science Direct, Google Scholar, and Scopus databases were used with the keywords ‘lipid emulsion/parenteral nutrition and platelets’. Due to limited research, all studies (1971–2022) conducted on human subjects (n = 51) were included, and studies on experimental animals were excluded.
The impact of parenteral nutrition lipid emulsions on platelets was divided into two headings that are (1) platelet function and (2) platelet count and coagulation. Further, studies were investigated by subheadings (1) soybean oil-based, (2) soybean oil/medium-chain triglycerides-based, (3) fish oil-based, (4) olive oil/soybean oil-based, (5) medium-chain triglycerides/fish oil-based, (6) soybean oil/medium-chain triglycerides/olive oil/fish oil-based. Due to insufficient literature, some subheadings were evaluated together (Fig. 2).

Fig. 2. Heat map of studies investigating parenteral nutrition lipid emulsion on platelets distributed to a. platelet function tests b. type of research. FO, fish oil; LCT, long-chain triglycerides; MCT, medium-chain triglycerides; OO, olive oil; PN, parenteral nutrition; SO, soybean oil.
Impact of PN lipid emulsions on platelet function, platelet count, and coagulation
Soybean oil-based lipid emulsion
Impact on platelet function
The most studied lipid emulsion in the literature in the context of platelets is soybean oil-based lipid emulsion (100% LCT) (Fig. 2). To start with, no change was reported in platelet aggregation in infants(Reference Herson, Block, Eisenfeld, Maderazo and Krause40) and adult patients(Reference Ball and Allington30,Reference Burnham, Cockbill, Heptinstall and Harrison41,Reference Porta, Planas, Padro, Pico, Valls and Schwartz42) with SO (100% LCT) lipid emulsion administration. Interestingly, it is known that SO lipid emulsions contain the precursor (linoleic acid) of arachidonic acid, which, even in low concentrations, might activate and induce platelets to undergo a release reaction and aggregate(Reference Ball and Allington30). Despite this, there was no clinical evidence of bleeding or thrombotic tendency in these patients receiving SO lipid emulsion(Reference Burnham, Cockbill, Heptinstall and Harrison41). Further, the lipid emulsions may also lead to increased plasma levels of non-esterified fatty acids (NEFA), associated with platelet aggregation(Reference Herson, Block, Eisenfeld, Maderazo and Krause40,Reference Burnham, Cockbill, Heptinstall and Harrison41) . In contrast, decreased platelet aggregation was reported in familial hypercholesterolaemia patients receiving SO (100% LCT) infusion(Reference Hayek, Fuhrman, Levy, Aviram and Brook36). Since enhanced platelet aggregation is seen in patients with accelerated atherosclerosis thus, inhibition of platelet aggregation points to potential anti-atherosclerotic effects of SO (100% LCT) and may be due to specific diseases and patients(Reference Hayek, Fuhrman, Levy, Aviram and Brook36,Reference Aviram and Deckelbaum37) (Table 2).
Table 2. Summary of the studies measuring the effect of soybean oil-based lipid emulsions on platelet function
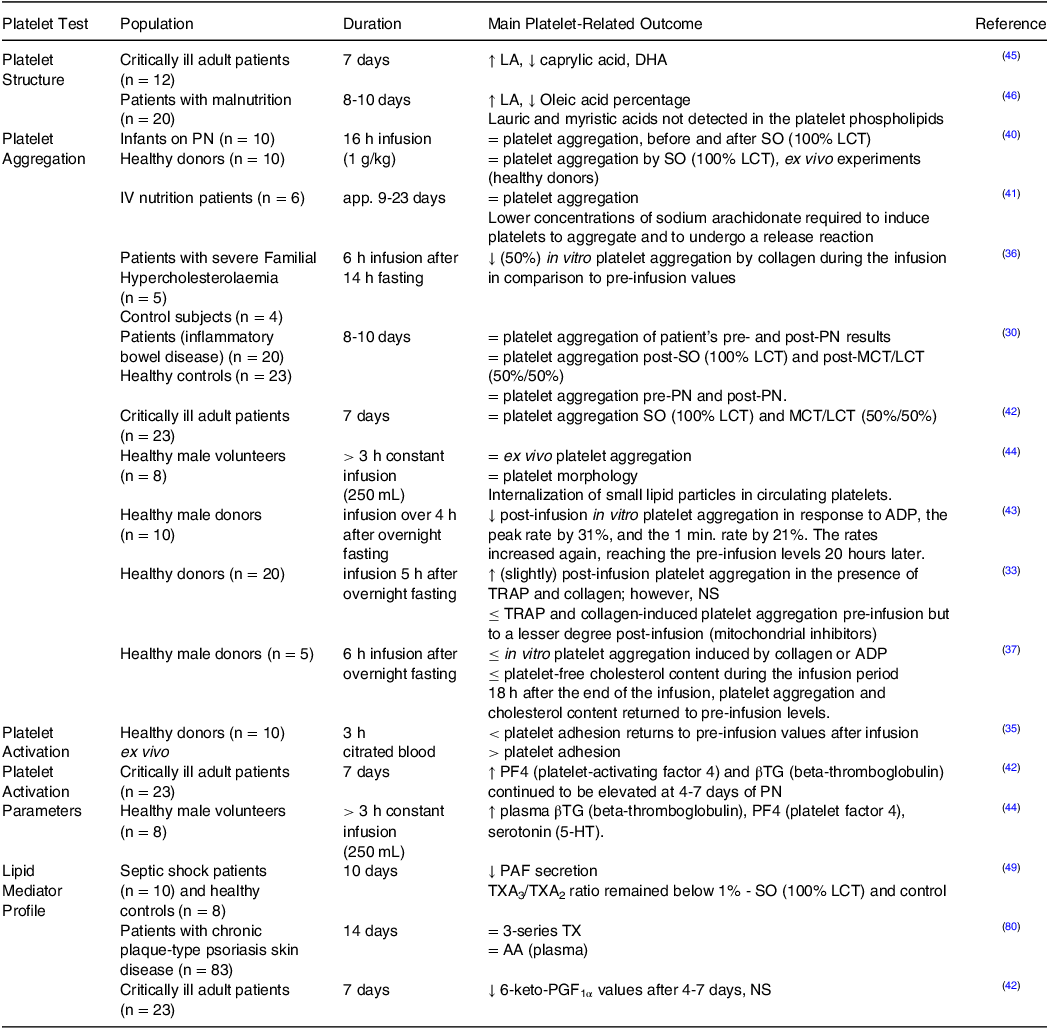
SO, soybean oil; LCT, long-chain triglycerides; LA, linoleic acid; DHA, docosahexaenoic acid; MCT, medium-chain triglycerides; ADP, adenosine diphosphate; TRAP, thrombin receptor activator peptide; NS, non-significant; PAF, platelet-activating factor; TX, thromboxane; FO, fish oil; AA, arachidonic acid; PG, prostaglandin.
↑, increase; ↓, decrease; =, no change; ≤, reduce.
Studies investigating healthy subjects have shown different results. Decreased platelet aggregation responses to agonists were also shown in healthy male donors(Reference Aviram and Deckelbaum37,Reference Wang, Xu and Gustafson43) due to SO (100% LCT) lipid infusion. These studies also indicated anti-atherogenic properties of SO (100% LCT) lipid emulsion due to reduced free cholesterol in mononuclear cells(Reference Wang, Xu and Gustafson43) and platelets(Reference Aviram and Deckelbaum37), which might decrease platelet aggregation. The liposomes and triglyceride-phospholipid particles in SO (100% LCT) were held responsible for the change in platelet cholesterol content and in vitro platelet aggregation(Reference Aviram and Deckelbaum37) as they can lead to the depletion of cholesterol in cells and blood vessel endothelium(Reference Wang, Xu and Gustafson43). Additionally, reduced platelet adhesion by SO (100% LCT) lipid emulsion infusion to healthy donors(Reference Kapp, Duckert and Hartmann35) was shown; however platelet adhesion increased ex vivo. This study concludes that a humoral factor can reduce platelet adhesion. The slight decrease in platelet adhesion concluded that this lipid emulsion should not be infused in cases with severe hypercoagulability. In general, this lipid emulsion is safe to use without a risk of hypercoagulability(Reference Kapp, Duckert and Hartmann35) (Table 2).
Other studies on healthy volunteers receiving SO (100% LCT) lipid infusion showed no change in platelet aggregation(Reference Beaulieu, Vitseva, Tanriverdi, Kucukural, Mick, Hamburg, Vita and Freedman33,Reference Jarnvig, Naesh, Hindberg, Behnke, Bregengaad and Wilhjelm44) . However, some subjects tended towards an increase in threshold values of platelet aggregation of some agonists, and there was a release of platelet-specific peptides such as βTG (beta-thromboglobulin), PF4 (platelet-activating factor 4), and 5-HT (5-hydroxytryptamine). The release of platelet products in the plasma and no sign of platelet activation under the electron microscope indicate different platelet subpopulations. Although normal platelet morphology was seen under the electron microscope, the lipid infusion showed internalized small lipid particles on the surface of platelets. These findings suggests that platelets are in close contact with lipid emulsion particles, which may induce changes in platelet surface properties, leading to electrochemical charge and rapid clearance of platelets from the circulation(Reference Jarnvig, Naesh, Hindberg, Behnke, Bregengaad and Wilhjelm44). Additionally, mitochondrial inhibitors used in one study have led to limited ATP production eventually, leading to reduced platelet aggregation because platelets do not have enough energy to undergo shape change and granule release. However, these inhibitors were less effective post-infusion of SO (100% LCT), which may indicate promotion of aggregation at some point. Thus, SO (100% LCT) lipid emulsion might increase platelet aggregation when mitochondria function is inhibited and can affect the transcript profile of platelets with up-or down-regulation of genes associated with cell motility, adhesion, cell cycle progression, and metabolism. Continuous exposure would significantly solidify these changes and negatively impact haemostasis by increasing the risk for thrombosis(Reference Beaulieu, Vitseva, Tanriverdi, Kucukural, Mick, Hamburg, Vita and Freedman33) (Table 2).
Accordingly, studies investigated the effects of PN lipid emulsions on platelet plasma membrane phospholipids. For instance, SO (100% LCT) lipid emulsion increased platelet LA and AA levels(Reference Planas, Porta, Sagristá, Mora, Padró and Picó45,Reference Ball46) and conversely decreased oleic acid, caprylic acid, and docosahexaenoic acid (DHA) levels(Reference Planas, Porta, Sagristá, Mora, Padró and Picó45). However, lauric acid and myristic acids were not detected in platelet phospholipids(Reference Ball46). These fatty acid changes in platelet membranes may be due to lipid emulsions’ exogenous fatty acid source(Reference Burnham, Cockbill, Heptinstall and Harrison41). Additionally, the platelet fatty acid profile may change following differences in the plasma fatty acids. After SO (100% LCT) lipid emulsion administration, increased plasma of AA(Reference Burnham, Cockbill, Heptinstall and Harrison41), LA, palmitoleic, and palmitic acid levels, and decreased oleic acid levels were reported(Reference Ball46) (Table 2).
The increased LA (precursor of AA), decreased DHA(Reference Planas, Porta, Sagristá, Mora, Padró and Picó45), α-linolenic acid (ALA), along with a decreased ratio of n-3/n-6 PUFA content of the platelet membrane(Reference Planas, Porta, Sagristá, Mora, Padró and Picó45), may also lead to platelet activation via eicosanoid synthesis(Reference van der Meijden and Heemskerk47,Reference Mitchell and Kirkby48) . Following the phospholipid fatty acid change in the platelet plasma membrane, the lipid mediator profile change is shown in a low TXA3/TXA2 ratio by SO (100% LCT)(Reference Mayer, Fegbeutel, Hattar, Sibelius, Kramer, Heuer, Temmesfeld-Wollbrück, Gokorsch, Grimminger and Seeger49), which may also lead to platelet activation and aggregation. Additionally, SO (100% LCT) increased PF4 and βTG in activated platelets(Reference Porta, Planas, Padro, Pico, Valls and Schwartz42,Reference Jarnvig, Naesh, Hindberg, Behnke, Bregengaad and Wilhjelm44) , indicating increased platelet activation (Fig. 2b).
Impact on platelet count and coagulation
Platelet count is a common blood test used in many studies investigating PN (Fig. 2a). Decreased platelet count, defined as thrombocytopenia, may increase risk of bleeding(Reference Paniccia, Priora, Liotta and Abbate20). Thrombocytopenia was reported in patients (including children) receiving SO (100% LCT) along with morphologically abnormal large platelets(Reference Gibson, Simons, Raik and Barton50), decreased platelet survival(Reference Goulet, Girot, Maier-Redelsperger, Bougle, Virelizier and Ricour51), and cholestatic liver disease(Reference Clayton, Bowron, Mills, Massoud, Casteels and Milla52). Decreased platelet counts over time, and a higher risk of bleeding were reported to be associated with children with intestinal failure-associated liver disease (IFALD) that received SO (100% LCT)(Reference Gura, Calkins, Premkumar and Puder53).
In other studies, SO (100% LCT) administration did not cause thrombocytopenia(Reference Beaulieu, Vitseva, Tanriverdi, Kucukural, Mick, Hamburg, Vita and Freedman33,Reference Kapp, Duckert and Hartmann35,Reference Spear, Spear, Cohen and Pereira54–Reference Velasco Benítez, Ladino Meléndez and Luna Medicis56) in adult patients(Reference Cohen, Dahms and Hays55), healthy donors(Reference Kapp, Duckert and Hartmann35), and term and preterm newborns(Reference Velasco Benítez, Ladino Meléndez and Luna Medicis56). It is important to note that this may be due to phytosterols in lipid emulsions, which correlate with high plasma phytosterol levels. Also, reducing lipid infusion improved platelet count and liver function tests in some patients(Reference Clayton, Bowron, Mills, Massoud, Casteels and Milla52). Further, high serum lipid levels may lead to fat overload syndrome with haematologic symptoms such as prolonged bleeding time and decreased platelet survival(Reference Herson, Block, Eisenfeld, Maderazo and Krause40). Interestingly, SO (100% LCT) administration may stimulate thrombocytopenia with more extended study duration periods(Reference Velasco Benítez, Ladino Meléndez and Luna Medicis56) (Table 3).
Table 3. Summary of the studies measuring the effect of different lipid emulsions on platelet count and coagulation

SO, soybean oil; LCT, long-chain triglycerides; AA, arachidonic acid; MCT, medium-chain triglycerides; DGLA, dihomo-gamma-linolenic acid; LA, linoleic acid; PN, parenteral nutrition; GPIIb/IIIa, integrin αIIbβ3; FO, fish oil; GPIb, glycoprotein Ib-V-IX; IV, TRAP, thrombin receptor activator peptide; ADP, adenosine diphosphate; OO, olive oil; IV, intravenous; PFA, platelet function analyser; PG, prostaglandin.
↑, increase; ↓, decrease; =, no change; ≤, reduce.
Coagulation tests are frequently used clinical tests to assess blood clotting function in patients(Reference Harris, Bazydlo and Winter57). The steps in the coagulation cascade are measured by prothrombin time (PT), partial thromboplastin time (PTT), and thrombin time (TT). They can be used to provide information about blood clotting and thrombosis; however, they cannot predict the occurrence of bleeding(Reference Harris, Bazydlo and Winter57). A study conducted with paediatric patients reported that administration of SO (100% LCT) PN significantly increased PT and PTT after 1 year follow-up. Nevertheless, coagulation factor V and fibrinogen remained in their usual range, which was attributed to vitamin K deficiency(Reference Dahlstrom, Goulet, Roberts, Ricour and Ament58). A case study reported prolonged bleeding time (PT, PTT) and haematemesis in a patient that received SO (100% LCT) PN, which was due to the developed fat overload syndrome of the high lipid dose; however, the exact aetiology of platelet dysfunction is not known(Reference Campbell, Freedman, Pencharz and Zlotkin59).
Platelet engraftment is defined as ‘the independence from platelet infusion for at least seven days, during which the platelet count is regularly measured’ (more than 20 × 109/L)(Reference Hutt, Kenyon and Babic60) and is known to be crucial for the success of transplantation(Reference Doescher, Casper, Kraemer, Kapels, Petershofen and Müller61). Platelet engraftment timing is a valid predictor of possible complications in transplantation patients(Reference Doescher, Casper, Kraemer, Kapels, Petershofen and Müller61), and PN is used to quicken engraftment in these patients(Reference Çetin, Arpaci, Dere, Turan, Öztürk, Kömürcü, Özet, Beyzadeoğlu, Kaptan, Beyan and Yalçin62,Reference Tavakoli-Ardakani, Neman, Mehdizadeh, Hajifathali, Salamzadeh and Tabarraee63) . Studies have shown that individualized PN compared to conventional PN(Reference Tavakoli-Ardakani, Neman, Mehdizadeh, Hajifathali, Salamzadeh and Tabarraee63) and SO (100% LCT) total PN compared to partial PN in patients with delayed platelet engraftment(Reference Çetin, Arpaci, Dere, Turan, Öztürk, Kömürcü, Özet, Beyzadeoğlu, Kaptan, Beyan and Yalçin62). These results suggest that SO (100% LCT) may protect against thrombocytopenia in transplant patients; however, this may be related to thrombopoiesis.
To conclude, many studies report that SO (100% LCT) lipid emulsion administration is safe(Reference Compagnoni, Schulzki, Thoeny and Reinhart13,Reference Beaulieu, Vitseva, Tanriverdi, Kucukural, Mick, Hamburg, Vita and Freedman33,Reference Kapp, Duckert and Hartmann35,Reference Herson, Block, Eisenfeld, Maderazo and Krause40,Reference Burnham, Cockbill, Heptinstall and Harrison41,Reference Spear, Spear, Cohen and Pereira54,Reference Li, Li, Wan, Xia, Wang and Li64) . Studies demonstrate the potential for increased platelet aggregation(Reference Beaulieu, Vitseva, Tanriverdi, Kucukural, Mick, Hamburg, Vita and Freedman33,Reference Jarnvig, Naesh, Hindberg, Behnke, Bregengaad and Wilhjelm44) , and this effect may be attributed to the modified fatty acid composition of the platelet plasma membrane(Reference Burnham, Cockbill, Heptinstall and Harrison41,Reference Planas, Porta, Sagristá, Mora, Padró and Picó45,Reference Ball46) . On the other hand, in other studies, it has been reported that SO (100% LCT) may reduce platelet aggregation(Reference Hayek, Fuhrman, Levy, Aviram and Brook36,Reference Aviram and Deckelbaum37,Reference Wang, Xu and Gustafson43) , which is associated with reduced levels of free cholesterol in platelets(Reference Aviram and Deckelbaum37). The phytosterols in SO (100% LCT) were found to be potentially responsible for thrombocytopenia, leading to bleeding. However, it is essential to note that the observed bleeding was primarily associated with other factors, including PN-related liver disease(Reference Clayton, Bowron, Mills, Massoud, Casteels and Milla52,Reference Gura, Calkins, Premkumar and Puder53) , developed fat overload syndrome reported in a case study(Reference Campbell, Freedman, Pencharz and Zlotkin59). Moreover, the SO (100% LCT) lipid emulsion should be applied carefully in paediatric patients at risk of bleeding(Reference Gura, Calkins, Premkumar and Puder53). In conclusion, the variability of in vitro methods used to test platelet activation(Reference Ball and Allington30), as well as differences in the amounts and durations of the lipid emulsions applied(Reference Beaulieu, Vitseva, Tanriverdi, Kucukural, Mick, Hamburg, Vita and Freedman33,Reference Herson, Block, Eisenfeld, Maderazo and Krause40) , makes it hard to compare all studies. Accordingly, given that exact aetiology of platelet dysfunction remains unknown, sensitive platelet function tests should be used to investigate SO (100% LCT) lipid emulsions.
Soybean oil/medium-chain triglycerides-based lipid emulsion
Impact on platelet function
Soybean oil/MCT-based lipid emulsions are frequently studied to compare their effects with SO (100% LCT) lipid emulsions (Fig. 2). A small number of studies have demonstrated that platelet aggregation did not change in patients receiving MCT/LCT (50%/50%) emulsions compared to LCT (SO (100% LCT))(Reference Ball and Allington30,Reference Porta, Planas, Padro, Pico, Valls and Schwartz42) . Because the lipid emulsion may lead to changes in the cell membrane fatty acid composition of platelets due to the different fatty acid composition, MCT/LCT (50%/50%) is theoretically proposed to affect platelets differently compared to LCT (SO (100% LCT))(Reference Ball and Allington30,Reference Porta, Planas, Padro, Pico, Valls and Schwartz42) . However, the length of the study might be an essential factor for the modification of platelet membranes, and short study periods might be associated with similar aggregation responses(Reference Porta, Planas, Padro, Pico, Valls and Schwartz42). Additionally, the platelet function analyser system (PFA-100) is a new standard to detect platelet dysfunction. It reports a closure time of an easily measured and formed platelet plug under high shear flow conditions(Reference Pradier, Portois, Malaisse and Carpentier65). A study in healthy male volunteers showed no difference in platelet function via the PFA-100 system due to the lipid emulsion MCT/LCT (50%/50%), indicating it is safe to use(Reference Pradier, Portois, Malaisse and Carpentier65) (Table 4).
Table 4. Summary of the studies measuring the effect of soybean oil/medium-chain triglycerides-based lipid emulsions on platelet function

SO, soybean oil; LCT, long-chain triglycerides; AA, arachidonic acid; MCT, medium-chain triglycerides; DGLA, dihomo-gamma-linolenic acid; LA, linoleic acid; PN, parenteral nutrition; GPIIb/IIIa, integrin αIIbβ3; FO, fish oil; GPIb, glycoprotein Ib-V-IX; IV, TRAP, thrombin receptor activator peptide; ADP, adenosine diphosphate; OO, olive oil; IV, intravenous; PFA, platelet function analyser; PG, prostaglandin.
↑, increase; ↓, decrease; =, no change.
Flow cytometry is used as a sensitive tool for platelet function(Reference Schmitz, Rothe, Ruf, Barlage, Tschope, Clemetson, Goodall, Michelson, Nurden and Shankey66); however, studies regarding the effects of parenteral lipid emulsions on platelet aggregation and expression of GPIb, GPIIb/IIIa, and P-selectin are limited. The upregulation of platelet membrane glycoproteins may be associated with coagulopathy induced by PN(Reference Li, Li, Wan, Xia, Wang and Li64). In vivo studies have shown that MCT/LCT (50%/50%) administration did not show any difference in the expression of platelet receptors GPIIb/IIIa(Reference Li, Li, Wan, Xia, Wang and Li64,Reference Pradier, Portois, Malaisse and Carpentier65,Reference Carpentier, Portois and Malaisse67) , P-selectin(Reference Pradier, Portois, Malaisse and Carpentier65,Reference Carpentier, Portois and Malaisse67) , fibrinogen(Reference Pradier, Portois, Malaisse and Carpentier65,Reference Carpentier, Portois and Malaisse67) , and GPIb(Reference Pradier, Portois, Malaisse and Carpentier65,Reference Carpentier, Portois and Malaisse67) against agonists ADP, collagen, and TRAP-6(Reference Carpentier, Portois and Malaisse67) in patients(Reference Li, Li, Wan, Xia, Wang and Li64) and healthy donors(Reference Pradier, Portois, Malaisse and Carpentier65,Reference Carpentier, Portois and Malaisse67) . However, platelet P-selectin expression of the PN group was significantly higher than that of the control group (healthy donors) following long-term PN with MCT/LCT (50%/50%)(Reference Li, Li, Wan, Xia, Wang and Li64). Thus, PN administration longer than 30 days induced the activation of platelet glycoproteins, which may be a risk factor for thrombogenesis(Reference Li, Li, Wan, Xia, Wang and Li64). The fact that there was no platelet activation in bolus IV lipid emulsion injections to healthy donors(Reference Pradier, Portois, Malaisse and Carpentier65,Reference Carpentier, Portois and Malaisse67) raises the idea that the metabolic state of patients and their potential response to PN lipid emulsion treatment may be an additional factor to keep in mind. Moreover, Stoetzer et al. demonstrated that MCT/LCT (50%/50%) lipid emulsion increased the GPIIb/IIIa and P-selectin expression while decreasing the GPIb expression in ex vivo studies involving healthy donors(Reference Stoetzer, Nickel, Weißig, Großheim, Scheinichen, Doll and Jüttner32). This study emphasizes that their findings cannot be interpreted into in vivo conditions; nonetheless, it highlights the potential for PN to interact with cellular coagulation processes(Reference Stoetzer, Nickel, Weißig, Großheim, Scheinichen, Doll and Jüttner32) (Table 4).
Similarly, the platelet membrane fatty acids were investigated in patients receiving lipid emulsions, including MCT/LCT (50%/50%). The MCT/LCT (50%/50%) lipid emulsion reduced AA(Reference Planas, Porta, Sagristá, Mora, Padró and Picó45,Reference Ball46) and palmitoleic acid(Reference Planas, Porta, Sagristá, Mora, Padró and Picó45), whereas it increased the percentage of linoleic acid(Reference Simoens, Deckelbaum, Massaut and Carpentier68), palmitic acid, SFAs(Reference Ball46), and dihomo-γ-linolenic acid (DGLA)(Reference Ball46) in platelets. Interestingly, platelet oleic acid content decreased, and lauric acid and myristic acid were not detected in platelet phospholipids(Reference Ball46). Additionally, plasma composition alterations were reported in response to MCT/LCT (50%/50%) lipid emulsion administration. In particular, there was an increase in the percentage of LA, palmitoleic, and palmitic acid, while oleic acid decreased (Table 4). Moreover, low levels of plasma lauric and myristic acids were reported following the MCT/LCT (50%/50%) administration(Reference Ball46), which is probably in response to the lipid source coconut oil(Reference Adolph, Heller, Koch, Koletzko, Kreymann, Krohn, Pscheidl and Senkal4) (Table 1).
Platelet-related mediators were analysed in patients treated with lipid emulsions MCT/LCT (50%/50%). A study reported that the release of prostacyclin (measured by stable metabolite 6-keto-PGF1α) decreased in patients receiving MCT/LCT (50%/50%), although the difference was not statistically significant(Reference Porta, Planas, Padro, Pico, Valls and Schwartz42). Additionally, the administration of MCT/LCT (50%/50%) to patients resulted in a continuous elevation of PF4 and βTG in activated platelets at 4-7 days of PN treatment; however, no significant difference was observed when compared to the lipid emulsion SO (100% LCT)(Reference Porta, Planas, Padro, Pico, Valls and Schwartz42). These activation markers may be affected by the oxygen radicals in critical patients and thus showed no alterations in platelet function(Reference Porta, Planas, Padro, Pico, Valls and Schwartz42) (Table 4).
Impact on platelet count and coagulation
A study involving patients receiving parenteral nutrition lipid emulsions MCT/LCT (50%/50%) or SO (100% LCT) revealed no significant difference in platelet counts between pre- and post-PN administration(Reference Ball and Allington30). Conversely, a study on children receiving long-term PN with either MCT/LCT (50%/50%) or SO (100% LCT) lipid emulsions reported a reduction in platelet count. Interestingly, normalization of platelet count occurred in some cases within the first month after suspension of lipid infusions. As a result, this study could not report a protective effect of MCT/LCT (50%/50%) emulsions against hepatic injury and cholestasis, which may develop with the long-term infusion of lipid emulsions even within lipid infusion rate limits(Reference Colomb, Jobert-Giraud, Lacaille, Goulet, Fournet and Ricour69). While lipid emulsion-induced thrombocytopenia may be an adverse effect in paediatric patients, a case study involving an adult patient showed thrombocytopenia following lipid emulsion MCT/LCT (50%/50%) administration, which may be because of the pre-existing malnutrition of the patient(Reference Liang, Yang and Ho70). On the other hand, both healthy donors and patients who received the lipid emulsion MCT/LCT (50%/50%) showed no change in the coagulation tests, including PT, PTT, fibrinogen, TT(Reference Li, Li, Wan, Xia, Wang and Li64). Furthermore, the comparison of MCT/LCT (50%/50%) and SO (100% LCT) lipid emulsions did not differ in coagulation parameters, including PT, PTT, and fibrinogen levels(Reference Demirer, Aydintug, Ustun, Turkmen, Tuzun, Simsek, Basaran, Çelebi and Demirer71) (Table 3).
Similarly, the lipid emulsion MCT/LCT (50%/50%) did not differ in platelet engraftment in patients with various haematologic malignancies who underwent a haematopoietic peripheral blood stem cell transplantation(Reference Demirer, Aydintug, Ustun, Turkmen, Tuzun, Simsek, Basaran, Çelebi and Demirer71). Thus, MCT/LCT (50%/50%) may protect against thrombocytopenia. However, it is also proposed that engraftment duration is shorter in well-nourished patients; therefore, PN instead of the lipid emulsion type may be therapeutic in the context of platelet engraftment(Reference Tavakoli-Ardakani, Neman, Mehdizadeh, Hajifathali, Salamzadeh and Tabarraee63) (Table 3).
In conclusion, MCT/LCT (50%/50%) lipid emulsion showed no risk of bleeding in patients(Reference Ball and Allington30,Reference Li, Li, Wan, Xia, Wang and Li64,Reference Demirer, Aydintug, Ustun, Turkmen, Tuzun, Simsek, Basaran, Çelebi and Demirer71) . Low platelet count was reported in children receiving long-term PN(Reference Colomb, Jobert-Giraud, Lacaille, Goulet, Fournet and Ricour69). Additionally, increased platelet GPIIb/IIIa(Reference Stoetzer, Nickel, Weißig, Großheim, Scheinichen, Doll and Jüttner32) and P-selectin(Reference Stoetzer, Nickel, Weißig, Großheim, Scheinichen, Doll and Jüttner32,Reference Li, Li, Wan, Xia, Wang and Li64) expression and decreased GPIb expression(Reference Stoetzer, Nickel, Weißig, Großheim, Scheinichen, Doll and Jüttner32) were shown. However, platelet aggregation(Reference Ball and Allington30,Reference Porta, Planas, Padro, Pico, Valls and Schwartz42) was not affected by the lipid emulsion MCT/LCT (50%/50%). The increased platelet activation may be due to ex vivo results(Reference Stoetzer, Nickel, Weißig, Großheim, Scheinichen, Doll and Jüttner32) or patient status(Reference Li, Li, Wan, Xia, Wang and Li64), as platelet reactivity did not change in other studies(Reference Pradier, Portois, Malaisse and Carpentier65,Reference Carpentier, Portois and Malaisse67) . The change in platelet membrane fatty acids as in reduced AA(Reference Planas, Porta, Sagristá, Mora, Padró and Picó45,Reference Ball46) ; however, increased SFAs percentage(Reference Ball46) and increased LA(Reference Simoens, Deckelbaum, Massaut and Carpentier68) may be a mechanism leading to platelet activation, however, the following production of platelet lipid mediators did not show any change in studies. Moreover, the stable proportion of n-3 and n-6 fatty acids in platelets(Reference Bohnert, Maurer, Calder, Pratschke, Thul and Muller72) may represent the underlying mechanism for the protective effects of MCT/LCT (50%/50%) during platelet activation. To conclude, although MCT/LCT (50%/50%) lipid emulsion was reported safe to use towards the risk of thrombosis and bleeding, its protective effects compared to SO (100% LCT) lipid emulsion are not clearly shown in studies.
Fish oil-based lipid emulsion
Impact on platelet function
Pure fish oil-based lipid emulsions were also investigated in the context of platelets (Fig. 2). It has been reported that PN consisting of FO (100%) lipid emulsion decreased the aggregation response of platelets in surgical patients(Reference Veljović, Mihajlović, Subota, Antunović, Jevdjić, Udovicić, Popadić and Vulović34). This result was particularly pronounced with collagen aggregation, and there was no risk of bleeding in these patients. Therefore, FO (100%) lipid emulsions may be used to inhibit platelet aggregation, providing a cardioprotective effect in patients undergoing open heart surgery. However, the risk of bleeding should be considered carefully. Moreover, a remarkable reduction in aggregation was reported in healthy subjects following administration of FO (100%) lipid emulsion(Reference Elmadfa, Stroh, Brandt and Schlotzer73). Following the 24-hour administration of FO (100%) infusion to healthy subjects, collagen-induced aggregation of platelets increased even higher than the starting value, whereas ADP-induced aggregation (20% reduction) remained the same(Reference Elmadfa, Stroh, Brandt and Schlotzer73). Thus, FO (100%) infusion may have potential use in patients with high thrombosis risk(Reference Elmadfa, Stroh, Brandt and Schlotzer73). In contrast, IV or oral administration of FO (100%) lipid emulsion to healthy subjects showed no change in platelet function as in platelet adhesion and aggregation tested via a PFA-100 system, which may be due to the short-term study period (15 days)(Reference Delodder, Tappy, Liaudet, Schneiter, Perrudet and Berger74) (Table 5).
Table 5. Summary of the studies measuring the effect of fish oil-based lipid emulsions on platelet function

FO, fish oil; EPA, eicosapentaenoic acid; DHA, docosahexaenoic acid; AA, arachidonic acid; IV, intravenous; FA, fatty acids; SUP, supplementation; PC, phosphatidylcholine; PE, phosphatidylethanolamine; SO, soybean oil; ADP, adenosine diphosphate; Pre-op, preoperative; Post-op, postoperative; TRAP, thrombin receptor activator peptide PFA, platelet function analyser; PAF, platelet-activating factor; TX, thromboxane; PRP, platelet rich plasma; PG, prostaglandin.
↑, increase; ↓, decrease; =, no change.
The anti-thrombotic effects of FO (100%) lipid emulsion are suggested to be related to the alterations in plasma membrane phospholipid fatty acid profile and consequent eicosanoid synthesis(Reference Veljović, Mihajlović, Subota, Antunović, Jevdjić, Udovicić, Popadić and Vulović34,Reference Elmadfa, Stroh, Brandt and Schlotzer73,Reference Delodder, Tappy, Liaudet, Schneiter, Perrudet and Berger74) . In particular, FO (100%) lipid emulsion showed increased EPA and DHA in platelet phospholipids/membrane(Reference Delodder, Tappy, Liaudet, Schneiter, Perrudet and Berger74–Reference Mayser, Mrowietz, Arenberger, Bartak, Buchvald, Christophers, Jablonska, Salmhofer, Schill, Krämer and Schlotzer80) along with decreased AA and increased n-3 PUFA/AA membrane ratios(Reference Grimminger, Fuhrer, Papavassilis, Schlotzer, Mayer, Heuer, Kiss, Walmrath, Piberhofer, Lübbecke and Krämer75). Moreover, a study demonstrated that patients receiving FO (100%) showed a substantial increase in EPA levels in both platelet phosphatidylcholine and phosphatidylethanolamine compared to SO (100% LCT)(Reference Roulet, Frascarolo, Pilet and Chapuis81). Additionally, plasma EPA and DHA levels increased several folds(Reference Grimminger, Fuhrer, Papavassilis, Schlotzer, Mayer, Heuer, Kiss, Walmrath, Piberhofer, Lübbecke and Krämer75,Reference Madsen, Christensen, Toft, Aardestrup, Lundbye-Christensen and Schmidt76) , surpassing AA(Reference Grimminger, Fuhrer, Papavassilis, Schlotzer, Mayer, Heuer, Kiss, Walmrath, Piberhofer, Lübbecke and Krämer75). Conversely, no change was reported in fatty acids palmitic, stearic, oleic, and linoleic acid(Reference Grimminger, Fuhrer, Papavassilis, Schlotzer, Mayer, Heuer, Kiss, Walmrath, Piberhofer, Lübbecke and Krämer75). Notably, a rapid increase in n-3 PUFA in platelet phospholipids was reported within 4 hours during haemodialysis (single dose of n-3 PUFA lipid emulsion)(Reference Madsen, Christensen, Toft, Aardestrup, Lundbye-Christensen and Schmidt76) and within 12 hours perioperative administration of FO (100%)(Reference Berger, Delodder, Liaudet, Tozzi, Schlaepfer, Chiolero and Tappy77). Meanwhile, with n-3 PUFA administration, the increase of n-3 PUFA in plasma phospholipids occurred within 48 hours(Reference Madsen, Christensen, Toft, Aardestrup, Lundbye-Christensen and Schmidt76). It is important to note that the rapid increase in platelet EPA and DHA was attributed to the exchange between plasma NEFA and platelet fatty acids(Reference Madsen, Christensen, Toft, Aardestrup, Lundbye-Christensen and Schmidt76) (Table 5).
The potential of lipid mediators has yet to be fully understood; moreover, their role in platelets in health and disease states encourage new lipidomic methodologies to scan and image lipid mediators(Reference O’Donnell, Murphy and Watson82). In a case study, the administration of IV FO (100%) resulted in an increase in the TXB3/TXB2 ratio in stimulated platelets during infusion(Reference Grimminger, Fuhrer, Papavassilis, Schlotzer, Mayer, Heuer, Kiss, Walmrath, Piberhofer, Lübbecke and Krämer75). Various studies involving patients or healthy subjects have demonstrated decreased blood TX and TXB2 levels and increased blood and platelet-generated TXA3 levels(Reference Mayer, Fegbeutel, Hattar, Sibelius, Kramer, Heuer, Temmesfeld-Wollbrück, Gokorsch, Grimminger and Seeger49,Reference Elmadfa, Stroh, Brandt and Schlotzer73) . Moreover, an increase in platelet TXB3 generation(Reference Mayser, Mrowietz, Arenberger, Bartak, Buchvald, Christophers, Jablonska, Salmhofer, Schill, Krämer and Schlotzer80) and elevated TXA3/TXA2 ratio have been observed(Reference Mayer, Fegbeutel, Hattar, Sibelius, Kramer, Heuer, Temmesfeld-Wollbrück, Gokorsch, Grimminger and Seeger49), while prostaglandin levels remained stable(Reference Elmadfa, Stroh, Brandt and Schlotzer73). Also, FO (100%) administration increased platelet-activating factors synthesis over time(Reference Mayer, Fegbeutel, Hattar, Sibelius, Kramer, Heuer, Temmesfeld-Wollbrück, Gokorsch, Grimminger and Seeger49). Together, these findings may indicate the influence of the alternative lipid precursor on central lipoxygenase and cyclooxygenase pathways. These findings suggest that some EPA-containing membrane lipid pool(s), providing precursor fatty acids to the metabolic pathways of eicosanoid formation, may be rapidly regulated in exchange with plasma EPA(Reference Mayser, Mrowietz, Arenberger, Bartak, Buchvald, Christophers, Jablonska, Salmhofer, Schill, Krämer and Schlotzer80) (Table 5).
Impact on platelet count and coagulation
Fish oil-based lipid emulsions have been investigated concerning the risk of bleeding (Fig. 2a) and have been reported safe to use, with no evidence suggesting an increased risk of coagulopathy or bleeding abnormalities(Reference Martindale, Berlana, Boullata, Cai, Calder, Deshpande, Evans, Garcia-de-Lorenzo, Goulet, Li and Mayer8). However, it is essential to recall that the studies are limited. Patients receiving PN formulations with FO (100%) + SO (100% LCT) or SO (100% LCT) were investigated, and no differences were observed in terms of platelet counts or platelet function (measured by RTG- p-time) between groups(Reference Heller, Fischer, Rössel, Geiger, Siegert, Ragaller, Zimmermann and Koch83). Alternatively, bleeding was reported in a patient with intestinal failure after 26 days of sole FO (100%) lipid emulsion administration, following a regimen of FO (100%) + SO (100% LCT) lipid emulsion. Besides, there was no evidence of clot formation, PTT was normal, and haemorrhage resolved on day 3 with clot formation at bleeding sites(Reference Dicken, Bruce, Samuel, Wales, Nahirniak and Turner84). It has been reported that preoperative IV FO (100%) did not induce a change in PTT among surgical patients(Reference Veljović, Mihajlović, Subota, Antunović, Jevdjić, Udovicić, Popadić and Vulović34). Given this data, it was suggested that the potential for bleeding complications from sole FO-based therapy in high-risk infants with liver disease should be reconsidered(Reference Dicken, Bruce, Samuel, Wales, Nahirniak and Turner84) (Table 3).
In contrast, a study showed that FO (100%) lipid emulsion had a lower risk of bleeding in children with IFALD compared to SO (100% LCT) lipid emulsion, which was associated with bleeding. In theory, FO-based lipid emulsions have an increased risk of bleeding due to their inhibitory effects in platelet adhesion and platelet-stimulated thrombin generation. However, this might be a weak effect due to this study. Nevertheless, due to the lack of data, there should be caution when using FO-based lipid emulsions, especially in paediatric patients at risk of bleeding(Reference Gura, Calkins, Premkumar and Puder53).
In conclusion, the demonstrated anti-inflammatory effects of fish oil endorse the FO-based lipid emulsions as advantageous components in PN(Reference Mahan and Escott-Stump2,Reference Wanten and Calder3,Reference Fell, Nandivada, Gura and Puder6,Reference Calder, Jensen, Koletzko, Singer and Wanten7,Reference Calder12,Reference Ren, Cong, Wang, Tang, Tian, Lin, Zhang and Tang85) . The proposed reduction in platelet aggregation(Reference Elmadfa, Stroh, Brandt and Schlotzer73) after FO (100%) administration may result from two possible mechanisms. Firstly, the inhibition of cyclooxygenase by n-3 PUFAs, leading to decreased synthesis of TXA2 from AA in platelets(Reference Veljović, Mihajlović, Subota, Antunović, Jevdjić, Udovicić, Popadić and Vulović34,Reference Dicken, Bruce, Samuel, Wales, Nahirniak and Turner84) . Secondly, it could be a result from the synthesis of TXA3 from EPA or a general reduction in total TX synthesis(Reference Mayer, Fegbeutel, Hattar, Sibelius, Kramer, Heuer, Temmesfeld-Wollbrück, Gokorsch, Grimminger and Seeger49,Reference Elmadfa, Stroh, Brandt and Schlotzer73) . In the end, leading to elevated levels of TXB3 (Reference Mayser, Mrowietz, Arenberger, Bartak, Buchvald, Christophers, Jablonska, Salmhofer, Schill, Krämer and Schlotzer80) and TXA3 (Reference Elmadfa, Stroh, Brandt and Schlotzer73), thus elevated ratios of TXB3/TXB2 (Reference Grimminger, Fuhrer, Papavassilis, Schlotzer, Mayer, Heuer, Kiss, Walmrath, Piberhofer, Lübbecke and Krämer75) and TXA3/TXA2 (Reference Mayer, Fegbeutel, Hattar, Sibelius, Kramer, Heuer, Temmesfeld-Wollbrück, Gokorsch, Grimminger and Seeger49). In addition, this may be supported by the changes in platelet membrane structure, characterized by an increase in EPA and DHA(Reference Grimminger, Fuhrer, Papavassilis, Schlotzer, Mayer, Heuer, Kiss, Walmrath, Piberhofer, Lübbecke and Krämer75,Reference Madsen, Christensen, Toft, Aardestrup, Lundbye-Christensen and Schmidt76,Reference Durieu, Vericel, Guichardant, Roth, Steghens, Drai, Josserand, Fontaine, Lagarde and Bellon79,Reference Roulet, Frascarolo, Pilet and Chapuis81) along with decreased AA in membrane phospholipids. Moreover, an increase in n-3 PUFA/AA membrane ratios in the platelet membrane was reported(Reference Grimminger, Fuhrer, Papavassilis, Schlotzer, Mayer, Heuer, Kiss, Walmrath, Piberhofer, Lübbecke and Krämer75). Alternatively, a very low AA source may potentially result in AA inhibition that has been found to block platelet shape change and granule secretion, thus decreasing platelet aggregation(Reference Dicken, Bruce, Samuel, Wales, Nahirniak and Turner84). However, there might be a temporary inhibition of platelet aggregation due to FO (100%) lipid emulsion; hence, it may be favourable in high thrombosis risk patients(Reference Elmadfa, Stroh, Brandt and Schlotzer73). However, the acute effects of n-3 PUFAs might not be mediated by platelet plasma membrane incorporation since there was no change in platelet function via the PFA-100 system(Reference Delodder, Tappy, Liaudet, Schneiter, Perrudet and Berger74), and other alternative lipid precursor pools are pointed out(Reference Mayser, Mrowietz, Arenberger, Bartak, Buchvald, Christophers, Jablonska, Salmhofer, Schill, Krämer and Schlotzer80). Additionally, the reported anti-platelet effects were not shown to cause bleeding risk in many studies(Reference Martindale, Berlana, Boullata, Cai, Calder, Deshpande, Evans, Garcia-de-Lorenzo, Goulet, Li and Mayer8,Reference Gura, Calkins, Premkumar and Puder53) , except a case report(Reference Dicken, Bruce, Samuel, Wales, Nahirniak and Turner84) indicating that this lipid emulsion should be carefully used in paediatric patients(Reference Gura, Calkins, Premkumar and Puder53). Although n-3 fatty acids may likely affect haemostasis(Reference Wachira, Larson and Harris86), The American Society for Parenteral and Enteral Nutrition states that there is no evidence to support that fish oil-containing lipid emulsions increase the risk of coagulopathy or bleeding abnormalities(Reference Martindale, Berlana, Boullata, Cai, Calder, Deshpande, Evans, Garcia-de-Lorenzo, Goulet, Li and Mayer8).
Olive oil/soybean oil-based lipid emulsion
Impact on platelet function
The evidence in the literature on the effects of OO/SO (80%/20%) lipid emulsion is limited, with only one ex vivo study related to the topic found (Fig. 2). Flow cytometry, a sensitive tool for platelet function(Reference Schmitz, Rothe, Ruf, Barlage, Tschope, Clemetson, Goodall, Michelson, Nurden and Shankey66), was used to examine the effects of OO/SO (80%/20%) lipid emulsion on platelet function. Previous research indicated that the stimulation of platelets of healthy donors by OO/SO (80%/20%) incubation did not influence GPIIb/IIIa, P-selectin, and GPIb receptor expression independent of lipid concentration and stimulation of ADP and TRAP-6(Reference Stoetzer, Nickel, Weißig, Großheim, Scheinichen, Doll and Jüttner32). A study suggests that olive oil-based lipid emulsion OO/SO (80%/20%) has superior anti-inflammatory effects when compared to the blend lipid emulsion MCT/OO/FO (30%/25%/15%)(Reference Buschmann, Poeschl, Braach, Hudalla, Kuss and Frommhold87). Therefore, its effects on platelets should be further investigated, including whether it may be used in patients with high thrombosis or bleeding risk (Table 6).
Table 6. Summary of the studies measuring the effect of olive oil/soybean oil-based lipid emulsions on platelet function

MCT, medium-chain triglycerides; LCT, long-chain triglycerides; OO, olive oil; SO, soybean oil; FO, fish oil.
=, no change.
Medium-chain triglycerides/fish oil-based lipid emulsion
Impact on platelet function
Studies investigating the lipid emulsion MCT/FO (50%/10%) are also limited in the literature (Fig. 2). In PN solutions, including MCT/FO (20%/4%) that were added to healthy subjects’ plasma in different concentrations of volume (4, 10, 25%), platelet aggregation was decreased at a concentration of 4%, and the pore closure time was prolonged, indicating platelet function inhibition(Reference Compagnoni, Schulzki, Thoeny and Reinhart13). Compared to the lipid emulsion MCT/LCT (50%/50%) in healthy male volunteers, both emulsions showed no difference in platelet function via the PFA-100 system. Although the overall closure time was lower than basal values by MCT/FO (80%/20%) and MCT/LCT (50%/50%), it is indicated that both lipid emulsions are safe to use in the context of platelet adhesion and aggregation(Reference Pradier, Portois, Malaisse and Carpentier65) (Table 5).
The platelet function after MCT/FO (80%/20%) lipid emulsion administration was further assessed using flow cytometry. Since MCT is a substrate for lipoprotein lipase hydrolysis, the MCT/FO (80%/20%) lipid emulsion is proposed to serve as a rapid source of released MCT and n-3 fatty acids from emulsion particles, which subsequently incorporate into platelet cell membranes(Reference Pradier, Portois, Malaisse and Carpentier65,Reference Carpentier, Portois and Malaisse67) . The n-3 fatty acid availability may be associated with decreased platelet aggregation and increased bleeding risk, which raises safety concerns regarding using MCT/FO (80%/20%) lipid emulsion(Reference Pradier, Portois, Malaisse and Carpentier65). A previous report indicated that MCT/FO (80%/20%) administration to healthy subjects resulted in a lower P-selectin expression on day two by collagen(Reference Pradier, Portois, Malaisse and Carpentier65). Nevertheless, after a bolus IV injection of MCT/FO (80%/20%) to healthy subjects, no significant changes in the expression of platelet receptors GPIIb/IIIa(Reference Pradier, Portois, Malaisse and Carpentier65,Reference Carpentier, Portois and Malaisse67) , P-selectin(Reference Pradier, Portois, Malaisse and Carpentier65,Reference Carpentier, Portois and Malaisse67) , fibrinogen(Reference Pradier, Portois, Malaisse and Carpentier65,Reference Carpentier, Portois and Malaisse67) , and GPIb(Reference Pradier, Portois, Malaisse and Carpentier65,Reference Carpentier, Portois and Malaisse67) were observed in response to agonists ADP, collagen, and TRAP-6(Reference Carpentier, Portois and Malaisse67). These bolus IV studies have concluded that MCT/FO (80%/20%) lipid emulsions are safe to use and may show cardioprotective effects. An ex vivo study using the MCT/FO (50%/10%) (0.6 mg/ml) lipid emulsion on healthy subjects increased the expression of GPIIb/IIIa and P-selectin and, by contrast, decreased the expression of GPIb, indicating platelet activation(Reference Stoetzer, Nickel, Weißig, Großheim, Scheinichen, Doll and Jüttner32). However, these results indicate that in vivo outcomes may differ (Table 7).
Table 7. Summary of the studies measuring the effect of medium-chain triglycerides/fish oil-based lipid emulsions on platelet function

MCT, medium-chain triglycerides; FO, fish oil; LA, linoleic acid; DHA, docosahexaenoic acid; AA, arachidonic acid; LCT, long-chain triglycerides; EPA, eicosapentaenoic acid; PN, parenteral nutrition; DPA, docosapentaenoic acid; DGLA, dihomo-gamma-linolenic acid; GLA, gamma-linolenic acid; IV, intravenous; ALA, α-Linolenic acid; GPIIb/IIIa, integrin αIIbβ3; GPIb, glycoprotein Ib-V-IX; TRAP, thrombin receptor activator peptide; ADP, adenosine diphosphate; OO, olive oil; SO, soybean oil; PFA, platelet function analyser.
↑, increase; ↓, decrease; =, no change; ≤, reduce.
The lipid emulsion MCT/FO (50%/10%) compared to MCT/LCT (50%/50%) emulsion increased EPA(Reference Carpentier, Portois and Malaisse67,Reference Simoens, Deckelbaum, Massaut and Carpentier68,Reference Bohnert, Maurer, Calder, Pratschke, Thul and Muller72,Reference Carpentier, Hacquebard, Portois, Dupont, Deckelbaum and Malaisse88) , DHA(Reference Bohnert, Maurer, Calder, Pratschke, Thul and Muller72), and DPA(Reference Bohnert, Maurer, Calder, Pratschke, Thul and Muller72), whereas decreased AA(Reference Carpentier, Portois and Malaisse67,Reference Simoens, Deckelbaum, Massaut and Carpentier68) , LA(Reference Carpentier, Portois and Malaisse67,Reference Bohnert, Maurer, Calder, Pratschke, Thul and Muller72) , DGLA(Reference Bohnert, Maurer, Calder, Pratschke, Thul and Muller72), γ-linolenic acid (GLA)(Reference Bohnert, Maurer, Calder, Pratschke, Thul and Muller72) in platelets. Also, other studies reported no significant effects on the levels of LA, DHA(Reference Simoens, Deckelbaum, Massaut and Carpentier68), ALA, and docosapentaenoic acid (DPA)(Reference Carpentier, Portois and Malaisse67) in platelet phospholipids. In addition, the MCT/FO (50%/10%) emulsion was found to have a greater plasma elimination in the NEFA concentration content compared to MCT/LCT (50%/50%). Accordingly, platelets are rapidly enriched with n-3 PUFAs after bolus IV MCT/FO (50%/10%) injection(Reference Carpentier, Hacquebard, Portois, Dupont, Deckelbaum and Malaisse88). Additionally, MCT/FO (50%/10%) lipid emulsion-treated patients showed reduced platelet-derived growth factor, along with medical treatment(Reference Arshad, Chung, Steward, Metcalfe and Dennison89) (Table 7).
Soybean oil/medium-chain triglycerides/olive oil/fish oil-based lipid emulsion
Impact on platelet function
The lipid emulsion MCT/OO/FO (30%/25%/15%) is discussed in the literature due to its varying lipid sources and different fatty acids (Fig. 2). However, there is a limited number of studies related to its effects on platelets. When compared to SO (100% LCT), the MCT/OO/FO (30%/25%/15%) lipid emulsion increased total n-3 PUFA, EPA, DHA levels, n-3/n-6 PUFA and EPA/AA ratios, decreased total n-6 PUFA, and did not change AA in plasma phospholipids in patients receiving PN for over five days. This change in plasma fatty acids was also reported as similar to changes in platelet phospholipid composition(Reference Grimm, Mertes, Goeters, Schlotzer, Mayer, Grimminger and Furst90). These findings may be attributed to its n-6:n-3 PUFA ratio, 2.5:1(Reference Raman, Almutairdi, Mulesa, Alberda, Beattie and Gramlich10) (Table 1). The plasma lipid mediator profile after PN, including MCT/OO/FO (30%/25%/15%), showed increased leukotriene B5 and not significantly decreased leukotriene B4 with increased plasma leukotriene B5/B4 ratio in the plasma, suggesting favourable anti-inflammatory effects. However, platelet responses to this environment were not investigated(Reference Grimm, Mertes, Goeters, Schlotzer, Mayer, Grimminger and Furst90). Thus, the proposed anti-inflammatory environment due to MCT/OO/FO (30%/25%/15%) lipid emulsion should be investigated in platelets and thrombus formation.
Impact of blend lipid emulsions on platelet count and coagulation
The blend lipid emulsion MCT/OO/FO (30%/25%/15%) has been investigated in the literature and is shown that it may have benefits over the first-generation lipid emulsion SO (100% LCT)(Reference Dai, Sun, Li, Ding, Su, Sun, Xue, Yan, Zhao and Wang91,Reference Jackson, White and Zalla92) ; however, platelet-related studies are limited (Fig. 2a). According to a case report, thrombocytopenia was observed in a paediatric patient, with short bowel syndrome and IFALD, receiving a very rapid infusion of PN including MCT/OO/FO (30%/25%/15%) lipid emulsion(Reference Hojsak and Kolacek93) (Table 3). It was reported that the lipid composition is also essential as fish oil-based lipid emulsions may not lead to the fat overload syndrome due to an n-6:n-3 PUFA ratio of approximately 2.5:1 in contrast to soybean oil lipid emulsions, which has a ratio of 7:1 (Table 1). Thus, this case study confirms the infusion rate’s importance before the solution’s lipid composition(Reference Hojsak and Kolacek93). On the other hand, the lipid emulsion MCT/OO/FO (30%/25%/15%) did not increase the risk of thrombocytopenia in long-term patients(Reference Klek, Chambrier, Singer, Rubin, Bowling, Staun, Joly, Rasmussen, Strauss, Wanten and Smith94,Reference Wu, Kuo, Chuang, Yeh, Yin, Li, Wang, Chen, Wang and Lin95) and did not change PT and PTT(Reference Wu, Kuo, Chuang, Yeh, Yin, Li, Wang, Chen, Wang and Lin95); thus, it was reported safe without increased coagulation risk and bleeding(Reference Klek, Chambrier, Singer, Rubin, Bowling, Staun, Joly, Rasmussen, Strauss, Wanten and Smith94,Reference Wu, Kuo, Chuang, Yeh, Yin, Li, Wang, Chen, Wang and Lin95) (Table 3). Moreover, the lipid emulsion MCT/FO (20%/4%) administered to healthy subjects at different concentrations of plasma did not change PT and PTT(Reference Compagnoni, Schulzki, Thoeny and Reinhart13) (Table 3), and OO/SO (80%/20%) lipid emulsion was not investigated in the literature on platelet count and coagulation (Fig. 2).
To conclude, the PN lipid emulsion MCT/FO (20-50%/4-10%) might decrease platelet aggregation, indicating platelet function inhibition; however, there was no change in PT and PTT(Reference Compagnoni, Schulzki, Thoeny and Reinhart13). In addition, platelet activation was reported by the increased receptor expression of GPIIb/IIIa and P-selectin and decreased GPIb(Reference Stoetzer, Nickel, Weißig, Großheim, Scheinichen, Doll and Jüttner32) ex vivo; however, no change of the expressions of GPIIb/IIIa, P-selectin, fibrinogen, and GPIb were shown in vivo (Reference Carpentier, Portois and Malaisse67). The observed increases in the levels of EPA(Reference Carpentier, Portois and Malaisse67,Reference Simoens, Deckelbaum, Massaut and Carpentier68,Reference Bohnert, Maurer, Calder, Pratschke, Thul and Muller72,Reference Carpentier, Hacquebard, Portois, Dupont, Deckelbaum and Malaisse88) , DHA(Reference Bohnert, Maurer, Calder, Pratschke, Thul and Muller72,Reference Carpentier, Hacquebard, Portois, Dupont, Deckelbaum and Malaisse88) , DPA(Reference Bohnert, Maurer, Calder, Pratschke, Thul and Muller72), as well as decreases in the levels of AA(Reference Carpentier, Portois and Malaisse67,Reference Simoens, Deckelbaum, Massaut and Carpentier68) , LA(Reference Carpentier, Portois and Malaisse67,Reference Bohnert, Maurer, Calder, Pratschke, Thul and Muller72) , DGLA, GLA(Reference Bohnert, Maurer, Calder, Pratschke, Thul and Muller72) and platelet-derived growth factor(Reference Arshad, Chung, Steward, Metcalfe and Dennison89) may explain the reduction in platelet aggregation in response to MCT/FO (20-50%/4-10%). Overall, different concentrations of MCT/FO administration in PN solutions were reported safe regarding platelet activation processes(Reference Pradier, Portois, Malaisse and Carpentier65). However, there are only limited studies to conclude that it may inhibit/increase platelet activation and platelet aggregation (Fig. 2a).
Furthermore, the literature lacks data about blend parenteral nutrition lipid emulsions OO/SO (80%/20%) and MCT/OO/FO (30%/25%/15%) on platelets. The lipid emulsion OO/SO (80%/20%) demonstrated no change in platelet activation assessed by flow cytometry and was reported as a safe formula to use(Reference Stoetzer, Nickel, Weißig, Großheim, Scheinichen, Doll and Jüttner32). Moreover, MCT/OO/FO (30%/25%/15%) lipid emulsion was also reported safe to use without increased coagulation risk and bleeding(Reference Klek, Chambrier, Singer, Rubin, Bowling, Staun, Joly, Rasmussen, Strauss, Wanten and Smith94,Reference Wu, Kuo, Chuang, Yeh, Yin, Li, Wang, Chen, Wang and Lin95) ; however, platelet activation and aggregation along with changes in platelet lipids and the potentially synthesized lipid mediators were not investigated. According to the limited literature, both fish oil blend lipid emulsions may be advantageous in patients who have a high risk of bleeding(Reference Compagnoni, Schulzki, Thoeny and Reinhart13,Reference Wu, Kuo, Chuang, Yeh, Yin, Li, Wang, Chen, Wang and Lin95) , yet this subject should be further investigated. The proposed anti-inflammatory effects of these blend lipid emulsions should be further investigated in platelets to define their protocol in patients with high thrombosis or bleeding risk (Table 6).
Conclusions and future directions
According to guidelines, lipid emulsions that are essential macronutrient components of parenteral nutrition solutions, are generally found safe to use in bleeding and thrombotic disease processes. Potentially, lipid emulsions may be responsible for platelet-related changes by altering the plasma and cell membrane fatty acid composition and further eicosanoid synthesis. This may lead to changes in the platelet plasma membrane cholesterol/phospholipid ratio, which is an important determinant of membrane fluidity. Altered membrane fluidity might change the permeability and behaviour of membrane-bound enzymes and the receptor activity of platelets. The cell membrane structure is reported to be modified by PN with lipid emulsions.
Further, the plasma levels of NEFA may, in turn, activate platelets directly, leading to bleeding or thrombotic tendency. The lipid emulsions may also contain fatty acids (arachidonic acid), which might activate platelets directly. Additionally, the development of fat accumulation as the fat overload syndrome with high serum lipid levels may result in haematologic symptoms such as prolonged bleeding time and decreased platelet survival.
The reviewed papers on parenteral nutrition lipid emulsions and their effects on platelets pointed out patients at risk of bleeding and platelet aggregation. A limited number of studies have mentioned that some lipid emulsions (SO (100% LCT), FO (100%)) were reported to lead to bleeding in patients, which may be associated with other parenteral nutrition or patient-related factors. However, these lipid emulsions should be used cautiously, especially in paediatric patients with parenteral nutrition-related liver disease. In this context, due to the risk of decreased platelet aggregation response by FO (100%) lipid emulsion, FO blend lipid emulsions may be advantageous. However, there is only a limited number of studies investigating their effects on platelets. The inconsistency of studies is principally due to the methodological differences of the studies (platelet function tests, in vivo/ex vivo study design), patient characteristics (adult/paediatric, age, comorbidities), etc.
Furthermore, the results from patients and healthy subjects exclude the status of critically ill patients and their affected haemostasis due to other pathological factors. However, the investigated studies enhance the assumption that lipid emulsions affect platelets differently. Studies investigating platelets and parenteral nutrition should be supported to minimize the adverse effects and to benefit from the potential protective effects of parenteral nutrition lipid emulsions on platelets.
To conclude, it is now well known that the membranes of platelets that provide substrates for enzymatic conversion, can change in health and disease states and are responsive to the diet. Additionally, dietary intake of lipids and fatty acids is an evolving area of research in many diseases and health. Thus, the in vivo nature of parenteral nutrition therapy benefits future studies to investigate the effects of dietary lipids and fatty acids on diseases, health, and human metabolism.
Abbreviations
AA: arachidonic acid; ADP: adenosine diphosphate; ALA: α-linolenic acid; DGLA: dihomo-γ-linolenic acid; DHA: docosahexaenoic acid; DPA: docosapentaenoic acid; EPA: eicosapentaenoic acid; FO: fish oil; GP: glycoprotein; GPIb: glycoprotein Ib-V-IX; GPIIb/IIIa: integrin αIIbβ3; IFALD: intestinal failure-associated liver disease; IV: intravenous; LA: linoleic acid; LCT: long-chain triglycerides; MCT: medium-chain triglycerides; MUFA: monounsaturated fatty acids; NEFA: non-esterified fatty acids; OO: olive oil; PFA: platelet function analyser; PN: parenteral nutrition; PS: phosphatidylserine; PT: prothrombin time; PTT: partial thromboplastin time; PUFA: polyunsaturated fatty acids; SO: soybean oil; TEG: tromboelastography; TG: triglyceride; TRAP: thrombin receptor activator peptide; TT: thrombin time; TX: thromboxane.
Funding statement
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Competing interests
The authors declare no competing interest.
Author contributions
B. K. contributed significantly to the design, screening of the articles, and writing of the manuscript. F. T. contributed significantly to the work’s conception, design, and critical writing and reviewed the manuscript. Both authors have read and approved the final manuscript.