Animal models have been used extensively in studies on the regulation of energy balance and the factors that underlie the development of obesity. Perhaps the most widely used has been the genetically obese ob/ob (Lepob/Lepob) mouse, the profound obesity of which results from a mutation in a single recessively inherited gene(Reference Bray and York1,Reference Zhang, Proenca and Maffei2) . This gene encodes the hormone leptin, which is expressed and secreted principally by white adipocytes(Reference Zhang, Proenca and Maffei2). Mutations in the leptin receptor gene are also associated with obesity, as evident in both the diabetic db/db (Leprdb/Leprdb) mouse and the fatty fa/fa (Leprfa/Leprfa) rat. Each of these rodents with leptin pathway mutations are hyperphagic and this is an important factor in their obesity(Reference Bray and York1,Reference Trayhurn3) . However, several types of study have indicated that the obese mutants are also more ‘metabolically efficient’ as a consequence of reduced energy expenditure(Reference Trayhurn3). The reduced expenditure has been attributed to decreased thermogenesis in brown adipose tissue(Reference Himms-Hagen4–Reference Trayhurn6) – underpinning the extensive interest in this tissue – the implication of which is that leptin is a thermogenic hormone(Reference Friedman7).
The view that reduced expenditure is a feature of obese animals with a leptin pathway mutation and that the hormone is thermogenic (directly or indirectly) has, however, recently been strongly challenged(Reference Fischer, Cannon and Nedergaard8). It is argued that the reported hypometabolism in ob/ob and db/db mice, and in fa/fa rats, comes ‘from a misleading calculation artefact resulting from expression of energy expenditure per gram of body weight and not per intact organism’(Reference Fischer, Cannon and Nedergaard8). We consider this proposition here, focusing on the ob/ob mouse as the key model, and conclude that reduced energy expenditure is an important factor in the initiation and development of obesity in leptin pathway mutants.
Energy expenditure and metabolic efficiency in ob/ob mice
In considering the energetics of obese models, it is important to distinguish between the causes and the consequences of obesity; the critical question is whether reduced energy expenditure is a causal factor. The energetics of ob/ob mice relative to their lean siblings will vary according to the circumstances, particularly the stage of development and degree of obesity, as well as whether they are hyperphagic at the time of measurement. Thus, for example, results from comparisons of adult lean and obese animals need to be interpreted carefully given the up to 3-fold difference in body weight and the elevated food intake of the obese.
There are three major lines of evidence that lead to the conclusion that leptin-deficient ob/ob mice have a reduced energy expenditure and that this is an important component in the development of their obesity: (i) milk intake in suckling animals; (ii) pair-feeding studies in young, weaned animals; and (iii) measurements of RMR (Fig. 1).
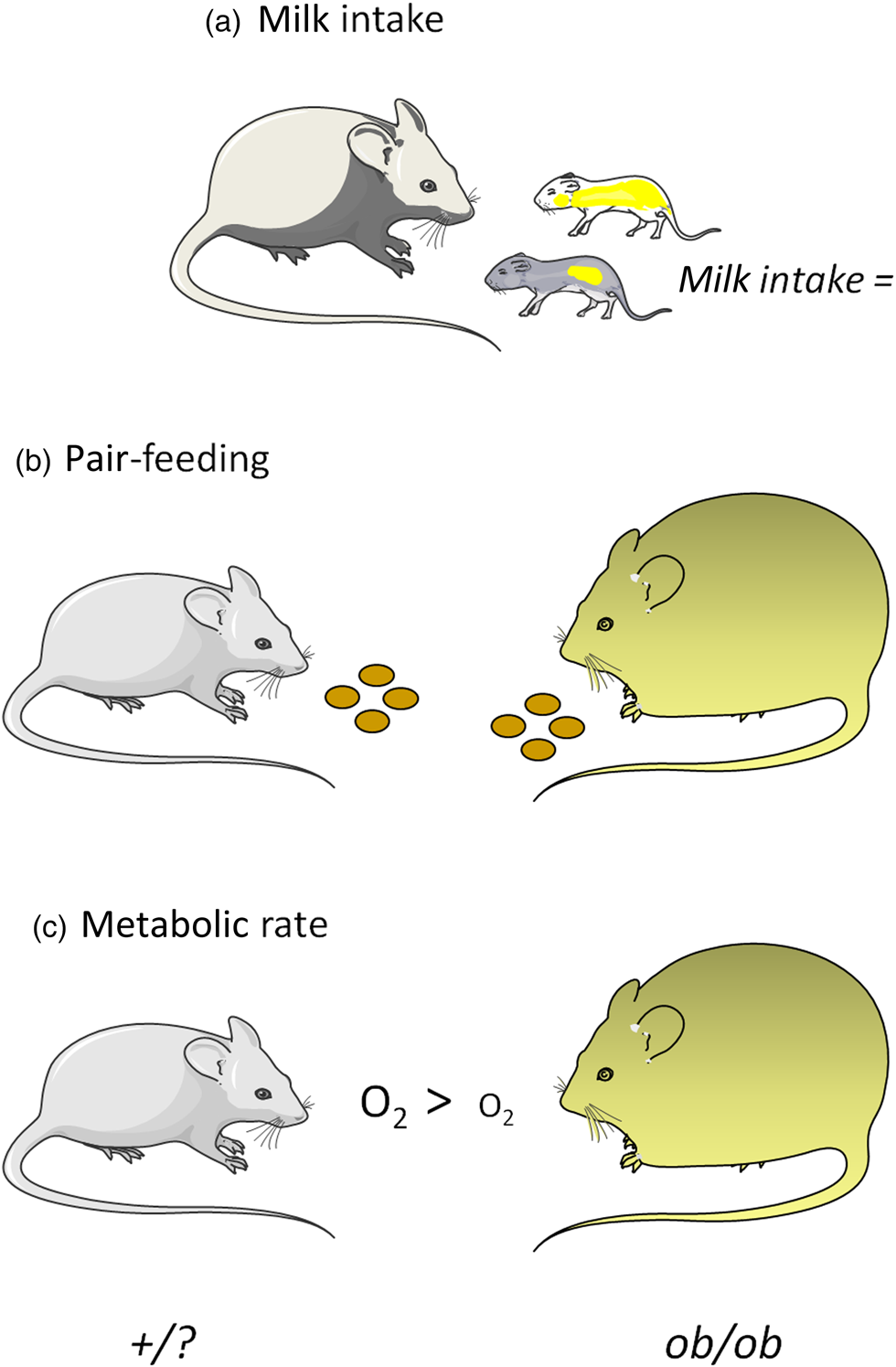
Fig. 1. Schematic representation of the main lines of evidence for increased metabolic efficiency, consequent to reduced energy expenditure, in ob/ob mice. (a) Milk intake in suckling ob/ob and +/? mice; (b) pair-feeding studies in which ob/ob mice were fed the ad libitum intake of lean siblings; (c) metabolic rate ‘per animal’ at temperatures below thermoneutrality. Mouse images are courtesy of Servier Medical Art (https://smart.servier.com).
It is important to note that ob/ob mice have been studied principally with the mutant gene on two different backgrounds – C57Bl/6J and the mixed ‘Aston’ background – although the ob allele has also been transferred to other backgrounds(Reference Fischer, Cannon and Nedergaard8). Aston strain mice are larger and were developed, and have been widely employed, in the UK.
Milk intake
Increased body fat is evident in ob/ob mice relative to their non-mutant +/? (+/+, ob/+) siblings before weaning in animals with the mutant gene on either the C57Bl/6J or Aston backgrounds(Reference Boissonneault, Hornshuh and Simons9,Reference Thurlby and Trayhurn10) . For example, with the mutant gene on the Aston background body fat was reported to be 20 % higher at 10 d and 72 % higher at 17 d of age than in mice bearing the lean genotype(Reference Thurlby and Trayhurn10).
Measurements of milk intake during the suckling period have shown that it is similar whether the individuals in a litter bear the ob/ob or +/? genotypes(Reference Lin, Romsos and Leveille11–Reference Contaldo, Gerber, Coward and Enzi13). This is the case if intake is assessed either through test weighing following re-feeding after a fast(Reference Lin, Romsos and Leveille11), or by more precise methods involving the administration of tritiated water to the dam(Reference Rath and Thenen14) or to the pups(Reference Contaldo, Gerber, Coward and Enzi13). Administration of tritiated water to the dams indicated that intake was not elevated in the ob/ob animals on the C57Bl/6J background at either 10 or 17 d of age(Reference Rath and Thenen14). Similarly, administering tritiated water to the pups, the most accurate of these three approaches, showed that intake in Aston ob/ob and +/? mice was the same between days 10 and 16 of life(Reference Contaldo, Gerber, Coward and Enzi13).
In each of these studies, milk intake was measured ‘per animal’ – it was not expressed relative to body weight. Thus, the evidence indicates that the initiation and early development of obesity in ob/ob mice occur without hyperphagia.
Pair-feeding
Several studies on the effect of food restriction on body weight and body fat of ob/ob mice post-weaning have been undertaken(Reference Fischer, Cannon and Nedergaard8). However, the most appropriate approach to examining metabolic efficiency is full energy balance experiments in which mutant mice are pair-fed to the daily ad libitum food intake of their lean siblings, with energy deposition being the measured end point.
Such full energy balance has been performed on Aston ob/ob mice pair-fed to the ad libitum intake of lean (+/?) siblings, employing the comparative slaughter technique (where the initial body composition of the experimental group is estimated by reference to a matched group of animals sampled at the start of the experiment)(Reference Thurlby and Trayhurn15). In this study, the ob/ob mice received their food in two daily meals in order to minimise any meal-feeding effects, the larger being presented just before the onset of the dark period. Both the rate of fat gain, and importantly the total energy gain, were higher in the obese mice than in the siblings to which they were yoked(Reference Thurlby and Trayhurn15). The study was conducted at several temperatures between thermoneutrality (32–33°C) and 17°C, and at each the energy deposition of the ob/ob was greater than that of the lean; at room temperature (23°C), the energy deposited was 2⋅3-fold higher in the obese than the lean(Reference Thurlby and Trayhurn15). Weight gain was lower in the obese, irrespective of environmental temperature, emphasising the importance of examining energy balance.
These energy balance studies clearly indicate that ob/ob mice exhibit a higher metabolic efficiency and can develop obesity in the absence of hyperphagia, reflecting a reduction in one or more components of energy expenditure. Interpretation of these observations is potentially confounded in that food restriction can induce torpor in mice(Reference Webb, Jagot and Jakobson16,Reference Himms-Hagen17) . However, torpor is not necessarily a factor in the enhanced metabolic efficiency of (Aston) ob/ob pair-fed to the habitual intake of lean siblings; most experiments demonstrating torpor have involved either fasting(Reference Webb, Jagot and Jakobson16,Reference Gavrilova, Leon and Marcus-Samuels18) , or feeding the mutant mice considerably less than the normal intake of the lean(Reference Himms-Hagen17,Reference Bolze, Morath and Bast19,Reference Skowronski, Ravussin and Leibel20) . Torpor is more likely to be evident with severe food restriction than with the limited restriction in the yoked pair-feeding studies.
Nevertheless, if torpor is a factor in the increased energy deposition of ob/ob mice relative to lean siblings when food is restricted in pair-feeding, then this does not of itself negate the concept of the mutants having an elevated metabolic efficiency. Rather, it provides a mechanistic basis with the decreased body temperature resulting in energy saving through reduced thermogenesis (and other components of expenditure).
A different form of pair-feeding was observed in a study in which mice were able to overfeed through the provision of a cafeteria diet(Reference Trayhurn, Jones and McGuckin21). Serendipitously, the energy consumption of the cafeteria-fed lean animals was similar to that of the ob/ob on their normal diet, so no food restriction was imposed on the obese. The energy gain and gross efficiency were both nearly 3-fold greater in the obese, consistent with impaired facultative diet-induced thermogenesis in the mutants(Reference Trayhurn, Jones and McGuckin21). Indeed, the apparent energy expenditure of the obese was <75 % of that of the cafeteria-fed animals.
Metabolic rate
A number of studies have measured RMR, or O2 consumption, in ob/ob mice compared with lean siblings. There are several important variables that need to be considered in interpreting the results, foremost of which is the index of body weight used to express expenditure. Energy expenditure is invariably lower in the obese when expressed ‘per g body weight’, as is commonly done, or on the basis of metabolic body size (body weight0⋅67/0⋅75)(Reference Mayer, Russell and Bates22–Reference Martins, Bargut and Aguila26). This is, however, unsurprising given that the obese animals may weigh up to three times more than their lean siblings, with the additional weight being due to white adipose tissue with its considerable quantity of TAG.
Expressing metabolic rate ‘per unit body weight’, or an index of metabolic size, is appropriate in comparing animals of widely different size – such as between species – but in energetic studies lean and obese siblings should be compared on a ‘per animal’ basis(Reference Fischer, Cannon and Nedergaard8,Reference Butler and Kozak27) . If expenditure is expressed ’per animal’ then in some studies it is reported to be lower in the obese than the lean, whether on Aston or C57Bl/6J backgrounds(Reference Thurlby and Trayhurn15,Reference Mayer, Russell and Bates22,Reference Arch, Ainsworth and Ellis28,Reference Wilson, Arch and Thurlby29) , while in others it appears not to be reduced(Reference Fischer, Cannon and Nedergaard8). There are several points to consider in interpreting these different results. First, if body weight is 2- to 3-fold greater, then RMR might be expected to be higher in order to meet the energetic costs implicit in the elevated body mass. Second, the hyperphagia of the obese animals leads to an increased energy cost associated with the processing of additional food – obligatory diet-induced thermogenesis. Thus, the failure to observe reduced energy expenditure in some studies may simply reflect the consequences of comparing very obese, hyperphagic mice with normal-weight lean siblings consuming less food.
Indeed, it can be argued that mature, hyperphagic obese mice should exhibit higher energy expenditure than their lean siblings and that it is anomalous that this is not always the case. The key reference is young lean and obese animals that do not exhibit large differences in body weight and food intake. This is evident in 18-d-old fa/fa rats where O2 consumption ‘per animal’ is lower since body weight is essentially the same as for lean siblings(Reference Kaplan30).
There are other variables between metabolic rate studies which may modify conclusions. These include environmental temperature and whether the animals were restrained or anaesthetised. With respect to environmental temperature, BMR, as measured by resting energy expenditure at thermoneutrality, is generally found to be similar, or even slightly elevated, in ob/ob mice ‘per animal’(Reference Fischer, Cannon and Nedergaard8). However, this does not indicate that they are not ‘hypometabolic’, or that the obesity is ‘entirely a result of their excessive food intake’, in contrast to what has been suggested(Reference Fischer, Cannon and Nedergaard8), but simply that there is no reduction in BMR.
RMR at the temperatures at which mice are usually maintained and studied – some 10°C below thermoneutral – will include the considerable energy costs of homoeothermy. For mice at 20–22°C, this is up to half their total resting energy expenditure(Reference Trayhurn and James31). And while the metabolic rate of ob/ob mice at thermoneutrality is not lower than that of the lean, generally the increase, whether expressed ‘per unit weight’ or ‘per animal’, as environmental temperature is lowered is less in the obese than the lean(Reference Hull and Vinter24,Reference Trayhurn and James31) .
Genetic background and mutant genes
The majority of studies on ob/ob mice have used animals with the mutant gene on the C57BL/6J background, while ‘Aston’ strain mice have been primarily used in the UK. Apparent differences between the two backgrounds have been emphasised, particularly in relation to whether energy expenditure is indeed lower ‘per animal’ in the mutants(Reference Fischer, Cannon and Nedergaard8). While it appears clear that reduced expenditure ‘per animal’ occurs in the Aston ob/ob strain, it is argued that this is not the case in those with the mutant gene on the C57Bl/6J background(Reference Fischer, Cannon and Nedergaard8) and that Aston mice represent an exception. However, as noted above, lower expenditure has been observed in C57Bl/6J ob/ob mice(Reference Arch, Ainsworth and Ellis28,Reference Wilson, Arch and Thurlby29) . Furthermore, similar milk intake is evident in suckling ob/ob mice of this background, with energy deposition being increased over the same period(Reference Rath and Thenen14).
It should be noted that reductions in energy expenditure (per animal) relative to lean animals are also exhibited by db/db mice on the C57BL/Ks background(Reference Trayhurn32). Energy balance studies in which young C57BL/Ks, or C57Bl/6J, db/db mice were pair-fed to the ad libitum food intake of their lean siblings also observed increased fat deposition and metabolic efficiency in the mice with the leptin receptor mutation, similar to ob/ob mutants(Reference Cox and Powley33,Reference Trayhurn and Fuller34) . We would argue, therefore, that Aston ob/ob mice are not anomalous, but broadly reflective of the characteristics of a leptin pathway mutation.
A further important model is the fa/fa rat which, like the db/db mouse, has a mutation in the leptin receptor gene. The general arguments relating to the mouse mutants also apply to the fa/fa rat. It is apparent that in suckling fa/fa rats, energy expenditure is reduced relative to the +/? siblings – although the data are expressed ‘per g body weight’, at this point body weight is similar in the different genotypes(Reference Kaplan30). Pair-feeding studies also indicate that the metabolic efficiency of fa/fa rats is greater than their lean siblings(Reference Bray, York and Swerloff35,Reference Pullar and Webster36) , and torpor is not known to occur in rats.
General comments
The analysis in this article supports the long-standing view that a reduction in energy expenditure is a component in the development of obesity in both ob/ob mice and leptin receptor mutants. The data in Aston strain ob/ob animals clearly demonstrate that: (i) the early development of obesity in suckling animals takes place without any increase in milk intake; (ii) young obese mutants pair-fed to the ad libitum intake of lean siblings exhibit an increased metabolic efficiency, consequent to reduced energy expenditure; and (iii) measurements of RMR indicate that it is lower ‘per animal’ in the obese than the lean at temperatures below thermoneutrality (Fig. 1). In the more widely employed mice with the leptin mutation on the C57Bl/6J background, milk intake data and our own studies on RMR(Reference Arch, Ainsworth and Ellis28,Reference Wilson, Arch and Thurlby29) paint a similar picture; we are not aware of full energy balance studies on C57Bl/6J ob/ob mice.
It is emphasised that in agreement with other authors(Reference Fischer, Cannon and Nedergaard8,Reference Butler and Kozak27) , it is important to present metabolic rate data ‘per animal’. It is also important to recognise that erroneous conclusions on the energetics of the obese may be made when based on hyperphagic animals weighing 2–3 times more than lean siblings consuming a normal amount of food. The central issue is causality – whether energy expenditure is reduced in younger ob/ob mice, underpinning the development of their obesity. As the animals become larger with substantial obesity, and are hyperphagic, energy expenditure would be expected to rise and may not necessarily be below that of the lean.
Leptin is well-recognised to regulate appetite, administration of the hormone leading to a reduction in food intake in leptin-deficient mutants(Reference Harris37). The generally accepted proposition is that leptin-deficient mice also exhibit a decrease in thermogenesis mediated by brown adipose tissue(Reference Himms-Hagen4–Reference Trayhurn6). It is now proposed that leptin is not a thermogenic hormone, but rather that it affects the regulation of body temperature through shifting thermoregulatory threshold and opposing torpor(Reference Fischer, Cannon and Nedergaard8). However, this would still indicate that leptin is thermogenic, albeit indirectly so. Alternative views include the recent proposal that leptin stimulates a hypothalamus–adrenal medulla–brown adipose tissue axis, thereby inducing lipolysis and increasing body temperature(Reference Perry, Lyu and Rabin-Court38).
In conclusion, we consider that the balance of evidence firmly supports the view that energy expenditure is reduced in ob/ob mice, and in leptin receptor obese mutants, and certainly so during the critical period of the early development of their obesity. This reduced expenditure has, of course, underpinned the interest in brown adipose tissue since the late 1970s(Reference Trayhurn3–Reference Trayhurn6).
Acknowledgements
The authors are in receipt of no specific grant from any funding agency, commercial or not-for-profit sectors, relevant to the present article.
Both authors conceived this review article, with P. T. drafting the manuscript, and with J. R. S. A. providing subsequent suggestions and comments.
The authors have no conflicts of interest.



