Introduction
Legume lectins represent a class of poorly recognised environmental toxicants. These sugar-binding proteins are difficult to destroy. In a recent large-scale study, the conditions required to inactivate lectin varied significantly, ranging from boiling for 15 min for black quinoa to 12 h of presoaking followed by boiling for 45 min for pinto beans.(Reference Adamcova, Laursen and Ballin1) Overnight soaking significantly reduced the boiling time to 30 min at 100°C (60 min for unsoaked beans) to destroy phytohemagglutinin (PHA) activity.(Reference Venter and Thiel2,Reference Pusztai and Grant3) Inadvertent exposure to these toxic sugar-binding proteins may occur when beans are consumed without adequate cooking, which may cause acute gastroenteritis characterised by abdominal pain, nausea, vomiting, and diarrhoea.(Reference Ramadass, Dokladny, Moseley, Patel and Lin4) However, little is known about the mechanism of the toxic effects of edible bean lectins. Most information on the cellular effects of legume lectin comes from ricin, the lectin of castor beans, which is not considered an edible legume.(Reference Spooner, Hart and Cook5) Ricin exerts its toxicity by preventing rRNA from binding to the protein elongation factor, resulting in the shutdown of protein synthesis.(Reference May, Yan and Tumer6) Whether or not lectins from edible beans also interfere with the function of newly formed proteins is not known.
PHA is a lectin of red kidney beans, a legume that is common in the Western diet. There are five different tetramer populations of PHA consisting of two polypeptide chains called E with erythroagglutinating and L with leucoagglutinating properties held together by noncovalent bonds.(Reference Nagae, Soga and Morita-Matsumoto7,Reference Banwell, Boldt, Meyers and Weber8) A variety of toxic effects has been reported with PHA, including impaired growth, reduced food intake, bacterial overgrowth, reduced intestinal brush border, injury to the intestinal mucosa, and alterations in general metabolism and the systemic immune system (thymus atrophy).(Reference Banwell, Boldt, Meyers and Weber8–Reference Pusztai, Clarke, Grant and King13) Interestingly, germ-free animals do not show the similar changes seen in conventional animals.(Reference Jayne-Williams and Hewitt14,Reference Jayne-Williams and Burgess15) In recent studies, no evidence of the toxicity of PHA lectin in rats was demonstrated. However, the growth of animals was slow, and the nitrogen fixation capacity of intestinal mucosal cells was impaired. In rats exposed to chow supplemented with uncooked red kidney beans, we have shown that intestinal barrier function is mainly impaired.(Reference Ramadass, Dokladny, Moseley, Patel and Lin4) Additionally, we documented greater total bacterial load in the liver, the proximal, mid, distal small intestine, and colon, and body weight loss. However, no differences in histological appearance were documented between the raw red kidney beans (RRKB)-fed group vs. control animals. The intestinal mucosa was histologically intact, and no increase in inflammatory cell levels was shown in the RRKB-fed group when compared to controls.(Reference Ramadass, Dokladny, Moseley, Patel and Lin4)
PHA has a broad modulatory effect on innate and adaptive immunity. In the peripheral circulation, lectins induce the production of antibodies, including immunoglobulins IgG, IgA, IgE, and IgM.(Reference Vojdani, Afar and Vojdani16,Reference Kumar, Verma and Sharma17) Apart from B cell activation,(Reference de Santana Brito, Ferreira and Klimczak18) subcutaneous injection of PHA induces T-cell mitogenesis and T-cell-mediated immunocompetence,(Reference Tella, Lemus, Carrete and Blanco19–Reference Maizel, Mehta, Hauft, Franzini, Lachman and Ford21) which requires the presence of monocytes-derived interleukin-6 (IL-6).(Reference Ceuppens, Baroja, Lorre, Van Damme and Billiau22) In the mouse macrophage cell line RAW264.7, PHA induced the secretion of tumour necrosis factor-α (TNF-α) and nitric oxide, indicating an increased inflammatory response.(Reference Kim, Yamasaki and Jiang23) Dietary lectins augmented IL-4 and IL-13 from human basophils.(Reference Haas, Falcone and Schramm24) The markers of cytotoxicity of immunocytes, the granzyme B, and perforin expression levels were elevated in tumour tissues of PHA-L-treated mice when compared with control mice.(Reference Wang, Leng and Duan25)
The inducible chaperone protein, heat shock protein 70 (HSP is 72 kDa in size in rodents but will be referred to as HSP70) contributes to the intestinal barrier function.(Reference Wu, Zhang and Yin26,Reference Akagi, Ohno, Matsubara, Fujimoto, Nakai and Inouye27) A key function of HSP70 is to protect newly expressed proteins from misfolding.(Reference Dokladny, Wharton, Lobb, Ma and Moseley28) Previous studies have shown decreased levels of stress proteins (HSP90 and HSP70) in rat gut and heat-stressed Cancer coli-2 (Caco-2) cells treated with PHA.(Reference Ovelgonne, Koninkx and Pusztai29) However, the relationship between lectin toxicity and the cellular chaperone machinery remains largely unknown. Given the relationship between lectin toxicity and protein elongation,(Reference Olsnes, Refsnes and Pihl30) we wondered whether lectins might exert their effects on chaperone activity.
Materials and methods
Animals
Twelve male Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA, USA) were used. The sample size was determined to achieve statistical power and analysis. No inclusion or exclusion criteria were used. The rats were housed in individual cages in the animal care facility under controlled temperature and humidity with a 12-hour light/12-hour dark cycle. The animals were given free access to water and standard rat chow (Harlan TEKLAD, TEKLAD Rodent Diet 8604, Madison, WI, USA) during a 7-day acclimatisation period. This study was approved by the Institutional Animal Care and Use Committee of the New Mexico Veteran Affair Health Care System, Albuquerque, NM (the date of approval and the Approval Number are 2007 and the Institutional Animal Care and Use Committee#07; the University of New Mexico036/Veteran Affair Health Care System#08_Henry Lin_07). In the present studies, all methods were performed in accordance with the relevant guidelines and regulations. We also confirm that the study is reported in accordance with ARRIVE guidelines.
Experimental design
Rats were randomised to a standard solid-pellet rat chow diet (Control, n = 6) or chow enriched with 26% raw red kidney beans (RRKB, n = 6).(Reference Banwell, Howard, Kabir, Adrian, Diamond and Abramowsky31) Red kidney beans were ground in a Waring blender (Waring Laboratory Science, Torrington, CT, USA), combined with ground standard rat chow, and formed into solid pellets. The test diets were fed ad libitum to rats for 15 d. Tissue specimens were collected during a non-survival surgery on day 15 of the test diet. Intestinal samples were collected from the mid-region of the small intestine and colon under 4% isoflurane general anaesthesia. The luminal contents were flushed with phosphate-buffered saline (PBS). Tissue samples were then collected in duplicate. One sample from each point of the collection was placed in RNA Later (Qiagen, Germantown, MD, USA) and stored at –80°C.
Chemicals
Culture media and related reagents were purchased from Life Technologies (Gaithersburg, MD, USA) and Sigma-Aldrich (Sigma-Aldrich Inc., St Louis, MO, USA). Transwell permeable filters were purchased from Corning (Corning Inc., NY, USA). PHA (Cat. # L8629) and FITC-labelled PHA (Cat. # 101097996) were purchased from Sigma-Aldrich and VWR, respectively. Anti-HSP70 (Cat. # ADI-SPA-812-D) antibodies were purchased from StressGen Biotechnologies (Victoria, British Columbia, Canada). Anti-β-actin was purchased from Sigma-Aldrich (Cat. # A2228). Secondary antibodies anti-rabbit IgG, HRP-linked (Cat. # 7074) were purchased from Cell Signaling Technology. Secondary antibodies anti-mouse IgG, HRP-linked (Cat. # 7076) were purchased from Cell Signaling Technology. The HSP70 Enzyme-Linked Immunosorbent Assay (ELISA) kit (Cat# ADI-EKS-700B) was purchased from Enzo Life Sciences (Farmingdale, NY, USA). The samples were analysed at 450 nm on the microplate reader (ELX 800, BIO-TEK Instruments). The sources of other chemicals are noted below.
RNA isolation and cDNA synthesis
Tissues were homogenised at 20 hertz for 60 s in a TissueLyzer II (QIAGEN GmbH, Hilden, Germany) in 180 µl of tissue lysis buffer using one 0.5 mm steel bead. Total RNA was extracted using RNeasy mini-Kit (QIAGEN GmbH, Hilden, Germany). cDNA was synthesised according to the kit manufacturer’s instructions (GeneAmp RNA PCR, Applied Biosystems, Grand Island, NY, USA). A mixture of random hexamers and oligo d(T) primers was used with multiscribe reverse transcriptase (Applied Biosystems, Grand Island, NY, USA). A Quantitative Polymerase Chain Reaction (QPCR) was performed using 100 ng of the synthesised cDNA as a template. The Polymerase Chain Reaction (PCR) amplification conditions are as follows: denaturation: 95oC for 15 min; denaturation: 95oC for 30 s, annealing: 60oC for 30 s, extension: 68oC for 45 s; 40 cycles.
Quantification of HSP70 and HSF1
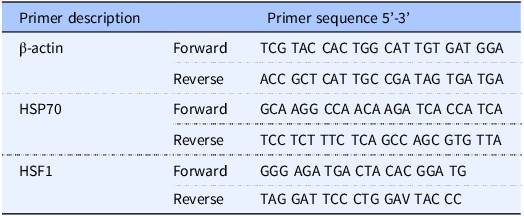
HSP70 and HSF1 genes were amplified using the primers listed in Table 1. Primers were purchased from Real Time primers, LLC (Elkins Park, PA, USA). The qPCR conditions were optimised for the different primers to achieve similar amplification efficiencies to compare different amplicons. Product specificity was tested by melting curves, and product size was visualised by electrophoresis on agarose gel. qPCR was performed using Quantitech SYBR Green PCR Kit (QIAGEN GmbH, Hilden, Germany) according to the manufacturer’s instruction based on a final volume of 25 μl that contains 0.5 µM of each primer using 100–150 ng of DNA as a template for each reaction. The thermal cycling conditions included the following: an initial denaturation step at 95°C for 15 min, 40 cycles of denaturation at 95°C for 30 s, annealing at 60°C for 30 s, an extension at 68°C for 45 s, and a plate read step. A melt curve analysis was then followed. The total qPCR amplification cycle threshold (CT) was used to represent the respective genes. CT values of HSF1 and HSP70 genes were normalised against CT values of the host housekeeping gene β-actin to generate a delta-delta CT value (relative CT value). Delta-delta CT values are inversely proportional to the total amount of gene expression so that a higher gene copy number is represented by a lower relative CT value.
Table 1. List of primers used
Cell culture
Caco-2 cell line was purchased from the American Type Culture Collection (Manassas, VA, USA) and maintained in Dulbecco’s Modified Eagle Medium (supplemented with 2 mM glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, and 10% foetal calf serum in a humidified atmosphere containing 5% CO2. Glutamine, penicillin, and streptomycin were purchased from GIBCO-BRL (Grand Island, NY, USA). Foetal calf serum was purchased from Atlanta Biologicals (Lawrenceville, GA, USA). Stock cultures were maintained in 100 mm dishes and were subcultured every 3–4 d. Cultures for all assays were maintained in 60 mm tissue culture plates and grown on Transwell filters following procedures as previously described.(Reference Dokladny, Wharton, Lobb, Ma and Moseley28)
Effect of PHA on transepithelial electrical resistance in Caco-2 cells
Transepithelial electrical resistance (TEER) of filter-grown Caco-2 cells was measured using an epithelial voltameter (World Precision Instruments, Saratoga, FL).(Reference Dokladny, Wharton, Lobb, Ma and Moseley28) Cells in short-term cultures were used for testing when the monolayer reached a resistance of at least 800 Ω/cm2 by 3–4 weeks. To study the effect of PHA on TEER, Caco-2 cells were exposed to PHA at the dose of 0 (control) or 200 μg/ml, and resistance readings were recorded at different time intervals (0, 12, 24, and 48 h). To study the effect of denatured lectin on TEER in Caco-2 cells, lectin was boiled at 100°C for 2 h.
Time course of entry of PHA into epithelial cells
Cellular localisation of lectin was assessed by immunofluorescent antibody labelling. Caco-2 monolayers grown on coverslips were exposed to lectin for increasing time points. At the end of the experimental period, Caco-2 monolayers were washed twice in cold PBS (4°C) and fixed with 2% paraformaldehyde for 20 min and mounted on microscope slides (Erie Scientific, Portsmouth, NH) using ProLong Gold Antifade Reagent within 4′,6-diamidino-2-phenyindole (DAPI) (Invitrogen Corp.). Immunolocalizations of lectin-FITC protein were visualised using a Nikon fluorescence microscope (Nikon, Garden City, NY, USA) equipped with a Hamamatsu digital camera (Hamamatsu Photonics, Hamamatsu, Japan). Images were processed with Wasabi software (Hamamatsu Photonics Deutschland, Herrsching, Germany).
Quantification of HSP70 by ELISA
Caco-2 cells grown in culture as monolayers were treated with 0 or 200 μg/ml of PHA for 48 h. Cells were washed with cold PBS, and clear cell extract was prepared by lysing the cells in an extraction buffer supplemented with protease inhibitor cocktail tablets (Complete Mini, Roche Diagnostics GmbH, Germany). Total protein content was measured by Bradford reagent (Cat. # 5000201; Quick StartTM from BIO-RAD). Clear cell extract was used for the quantification of HSP70 by ELISA. HSP70 ELISA analysis with each sample run in duplicates was performed per the kit manufacturer’s instruction (HSP70 ELISA kit – ADI-EKS-700B, Enzo Life Sciences, Farmingdale, USA) manual. The extraction buffer was supplemented with protease inhibitor cocktail tablets (Cat. # 11836170001; Complete Mini, Roche Diagnostics purchased from Sigma-Aldrich).
Western blot analysis of HSP70
Western blot analysis was performed to study the effect of lectin on HSP protein expression.(Reference Dokladny, Wharton, Lobb, Ma and Moseley28) Briefly, Caco-2 monolayers were exposed to PHA at a dose of 0, 100, or 200 μg/ml for 48 h. At the end of the experimental period, cells were immediately rinsed with ice-cold PBS and lysed with lysis buffer (50 mM Tris·HCl, pH 7.5, 150 mM NaCl, 500 μM NaF, 2 mM EDTA, 100 μM vanadate, 100 μM PMSF, 1 μg/ml leupeptin, 1 μg/ml pepstatin A, 40 mM paranitrophenyl phosphate, 1 μg/ml aprotinin, and 1% Triton X-100) and scraped. The cell lysates were placed in microfuge tubes and centrifuged using Eppendorf Centrifuge 5425R at 10,000 rpm for 10 min to yield a clear lysate. A clear supernatant was collected and stored at –20°C for further processing. Protein measurement was performed using Bio-Rad Protein Assay kit (DC Protein Assay, Bio-Rad Laboratories, Cat. # 5000111), and absorbances were read at 750 nm using a microplate reader (ELX 800, BIO-TEK Instruments). Laemmli gel loading buffer (Cat. # 1610737) was added to the lysate containing 20 μg of protein and boiled for 7 min. The samples were loaded and separated on an SDS-PAGE gel (Cat. # XP04202BOX; Invitrogen). The separated proteins were transferred from the gel to the nitrocellulose membrane (Trans-Blot Transfer Medium, Nitrocellulose Membrane, Cat. # 1620115; Bio-Rad Laboratories) overnight in a cold room at 4°C. The membrane was transferred from the apparatus and incubated for 2 h in a blocking solution (5% dry milk from Bio-Rad Cat. # 1706404 in TBS-Tween 20 buffer (Tween 20, Cat. # P9416 from Sigma-Aldrich) at room temperature with mild agitation. Then, the membrane was incubated with appropriate primary antibodies (see Materials and Methods, Chemicals Section for antibody specifications). Anti-HSP70 antibodies were used at 1:1000 dilution, and anti-β-actin was used at 1:2000 dilutions in a blocking solution overnight in a cold room at 4°C. After being rinsed three times (each wash for 10 min) in TBS-Tween buffer, the membrane was incubated (1 hour at room temperature) in appropriate secondary antibodies (see Materials and Methods, Chemicals Section for antibody specifications). A membrane was rinsed three times in TBS-Tween buffer (10 min each rinse) and developed using the Santa Cruz Western Blotting Luminol Reagents (Cat. # sc-2048, Santa Cruz Biotechnology, Santa Cruz, CA) on the Kodak BioMax MS film (Fisher Scientific, Pittsburgh, PA) using a film processor (Konica SRX-101A).
Effect of PHA and HSP70 on protein folding using Luciferase assay
Since proper protein folding of the enzyme luciferase is required for luminescence, reduced luciferase activity is seen with protein misfolding.(Reference Cashikar, Duennwald and Lindquist32) Caco-2 cells were transfected with constructs driving luciferase (pGL3) and incubated for 24 h to allow for optimal luciferase expression. For reporter gene assays testing HSP70 gain-of-function, pGL3 vector was co-transfected with either the control virus (AdTrack) or the virus driving HSP70 protein expression (Ad70). Following incubation with PHA at the dose of 0 (control) or 200 μg/ml at 37°C for 48 h, cells were washed twice with 1 ml of ice-cold PBS, lysed, incubated at room temperature for 15 min, scraped, and placed in microfuge tubes. A clear lysate was then obtained by centrifuging at 13,000 rpm for 20 s. Twenty microliters of the supernatant were assayed for luciferase activity using the luciferase assay system (Promega, Madison, WI). Luciferase values were determined by a Lumat LB9501 luminometer (Berthold, Wildbad, Germany).
Statistical analysis
To determine the sample size for TEER experiments in Caco-2 cells, we used an online Computing Two Means – Sample Size calculator at https://select-statistics.co.uk/calculators/. The confidence level was set at 95% and power at 80%. Based on our previous studies,(Reference Ramadass, Dokladny, Moseley, Patel and Lin4) we estimated the difference between the untreated and treated groups at 20 and the variance at 70. The estimated sample size was 3. To determine the sample size for experiments of gene expression, we used the preliminary data and set the difference between treated and untreated groups at 5 and variance at 9. The estimated sample size was 6. Non-parametric data were analysed using the non-parametric Wilcoxon rank-sum test, and figures were generated in the statistical package R or licensed GraphPad Prism 6. Data represent mean ± SD.
Results
PHA lectin reduced transepithelial electrical resistance (TEER) in Caco-2 cells
The effect of lectin on TEER was examined over 48 h (Fig. 1A). In control monolayers, there was a small (1–5% from the baseline) but not statistically significant increase when compared to the baseline in TEER at the early time points (for control monolayers, P = 0.7 baseline vs. 12-hour time point and P = 0.24 baseline vs. 24-hour time point). Although the causes of those increases remain unclear, they may be related to the fresh media added at time 0. When compared to TEER of control cells (854.3 ± 19.7) cultured in complete growth media, 200 μg/ml PHA treatment did not alter TEER (P = 0.68) after 12 h of treatment with PHA (873.3 ± 25.5). However, TEER of the PHA-treated cells dropped significantly in a time-dependent manner after 24 and 48 h, 773.6 ± 23.1 (P < 0.05) and 578.3 ± 15.4 (P < 0.0005), respectively. Since intense cooking is required to destroy legume lectins, we studied whether heat-denatured (100°C for 2 h) lectin PHA would affect transepithelial resistance in Caco-2 cells. Heat-denatured lectin PHA had no significant effect (P = 0.77; at 48-h time-point control monolayers vs. heat-denatured lectin-treated monolayers) on transepithelial resistance in Caco-2 cells, indicating that only intact protein can change the epithelial barrier integrity (Fig. 1B).
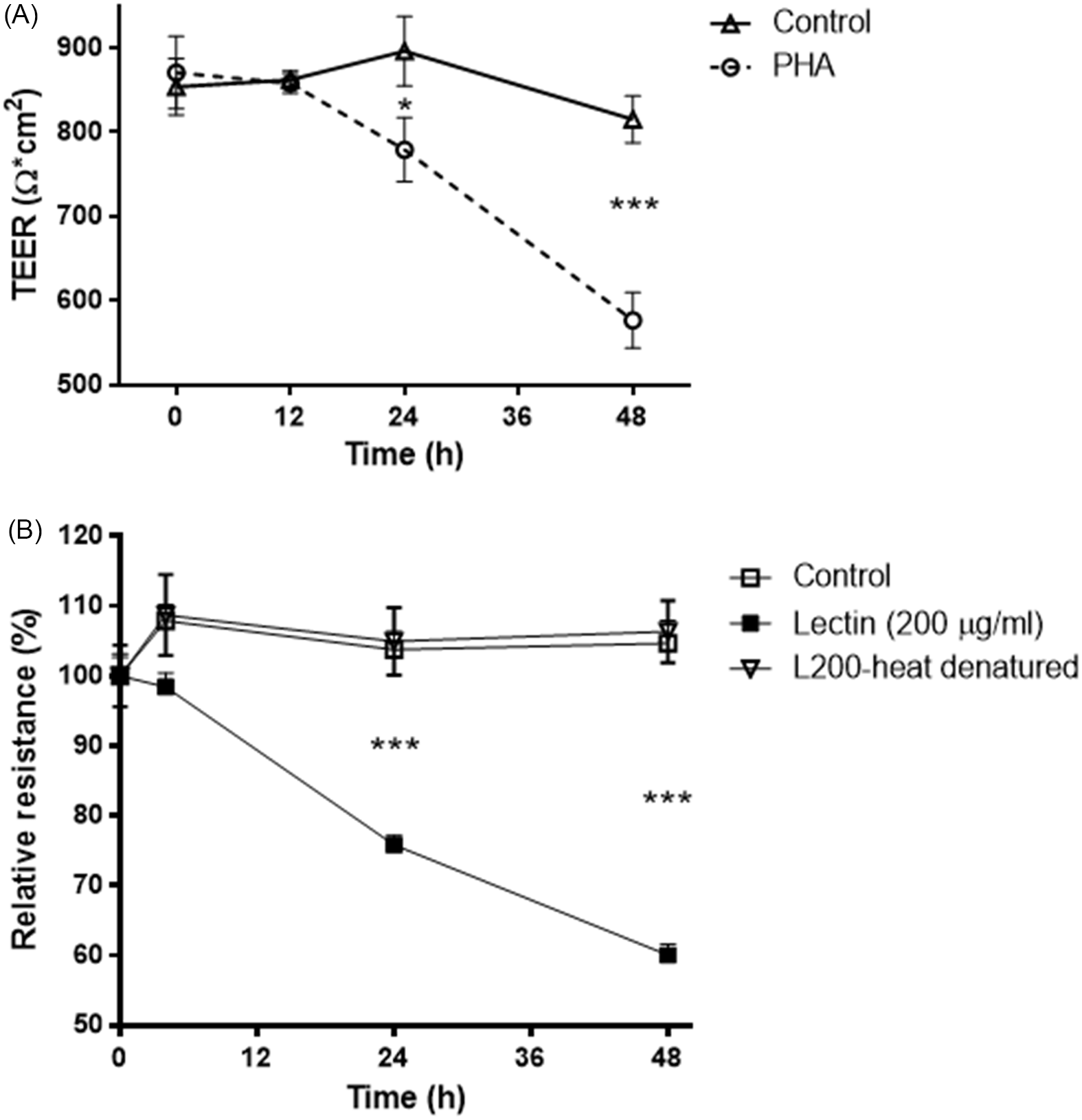
Fig. 1. A: Time-course effect of legume lectin PHA (200 μg/ml) on transepithelial electrical resistance in Caco-2 cells. * P < 0.05; *** P < 0.001. Values are means ± SD. n = 3 per group. B: The effect of heat-denatured (100°C for 2 h) lectin PHA (200 μg/ml) on changes of transepithelial resistance in Caco-2 cells. *** P < 0.001. Values are means ± SD. n = 3 per group.
Uptake of FITC-labelled PHA by Caco-2 cells
Caco-2 cells were exposed to PHA (200 µg/ml) conjugated with FITC across increasing time points. Uptake of FITC-labelled lectin by the cells was seen as soon as 1 hour after treatment (Fig. 2). During the initial time point, lectin fluorescence concentrated in the cytosol, and around the nucleus appeared as small dots. At the 3-hour time point, lectin intensity increased significantly in the cytoplasm and around the nucleus. Similarly, at 24- and 48-hour time points, lectin was present throughout the cytosol clustered around the nucleus. This data indicates that lectin enters the cell very quickly, and the process of entering the cells progresses over time.
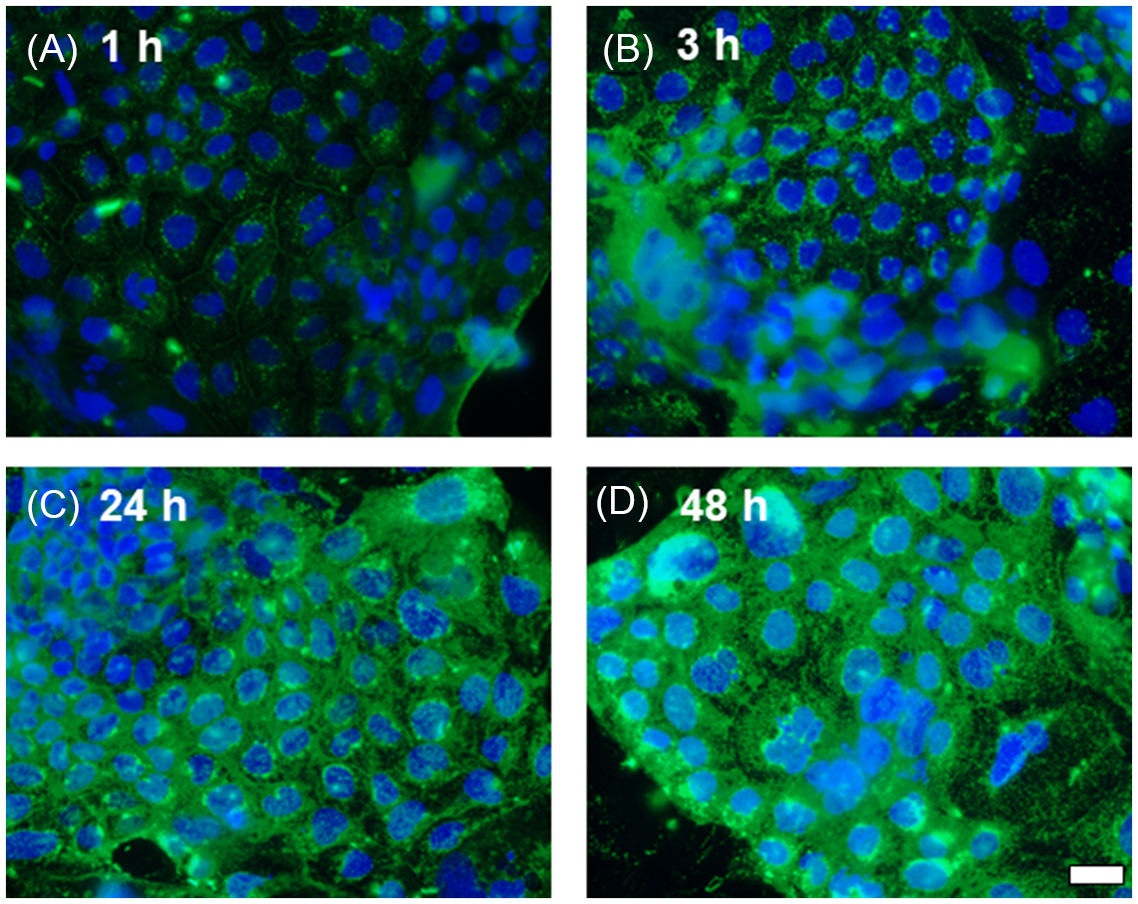
Fig. 2. Time course of legume lectin entry into Caco-2 epithelial cells. Lectin-FITC protein (green). Nuclei were visualised with DAPI (blue). A: 1-hour exposure. B: 3-hour exposure. C: 24-hour exposure. D: 48-hour exposure. Scale bar 20 μm.
Lectin PHA reduced intracellular HSP70 levels in Caco-2 cells
Protein estimation by an enzyme-linked immunosorbent assay (ELISA) on Caco-2 cell lysates treated with 200 μg/ml lectin PHA for 48 h showed a significant decrease (P < 0.05) of HSP70 levels (2.43 ± 0.95 ng/ml) compared to control cells grown in the absence of PHA (6.03 ± 0.56 ng/ml) (Fig. 3A). Western blot analysis of HSP70 levels in Caco-2 treated with lectin PHA (100 and 200 μg/ml for 48 h) confirmed these results (Fig. 3B).
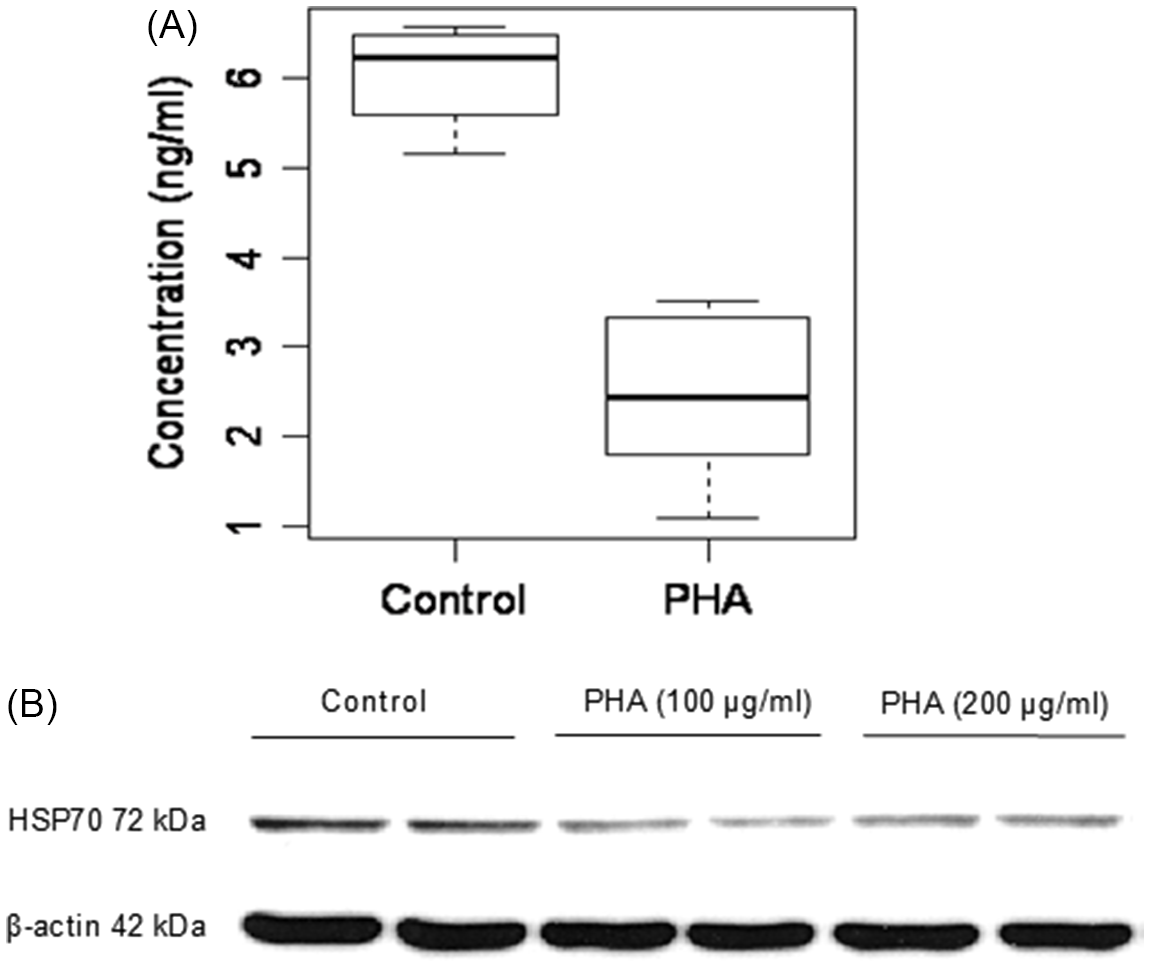
Fig. 3. The effect of lectin PHA (200 μg/ml for 48 h) on HSP70 protein expression by ELISA (A) and Western blot analysis (B) in Caco-2 cells. A: Values are means ± SD (n = 3). P < 0.05 Control vs. PHA.
PHA-impaired protein folding in an HSP70-reversible fashion
The effect of the lectin PHA (200 µg/ml) on protein folding was tested by measuring luciferase activity (decreased luciferase activity indicates greater protein misfolding). Cultures of Caco-2 cells were transfected with either pGL3 basic vector encoding the luciferase gene or co-transfected with constructs driving luciferase (pGL3) and HSP70 protein expression (Ad70). After a 24-hour incubation to allow for optimal luciferase expression, cells were subjected to PHA (200 µg/ml) treatment for 48 h following a measurement of luciferase activity. Controls were pGL3 single transfected cells. Compared to Controls (2.43 ± 0.16), the luciferase activity in Ad70 single transfected cells was not different (2.62 ± 0.38; P = 0.39 compared with control cells). Exposure to 200 μg/ml of PHA resulted in a significant decrease (2.07 ± 0.11; P < 0.01; compared with control cells) in luciferase activity in pGL3 single transfected cells, whereas in cells co-transfected with pGL3 and Ad70 (HSP70+PHA 200 μg/ml), this effect of PHA was abolished (2.66 ± 0.46; P = 0.4; compared with control cells. P < 0.05 compared with PHA-treated group). Thus, HSP70 overexpression inhibited the PHA-induced decrease in luciferase activity (Fig. 4). These data suggest that HSP70 exerts a protective effect against PHA-induced protein misfolding.
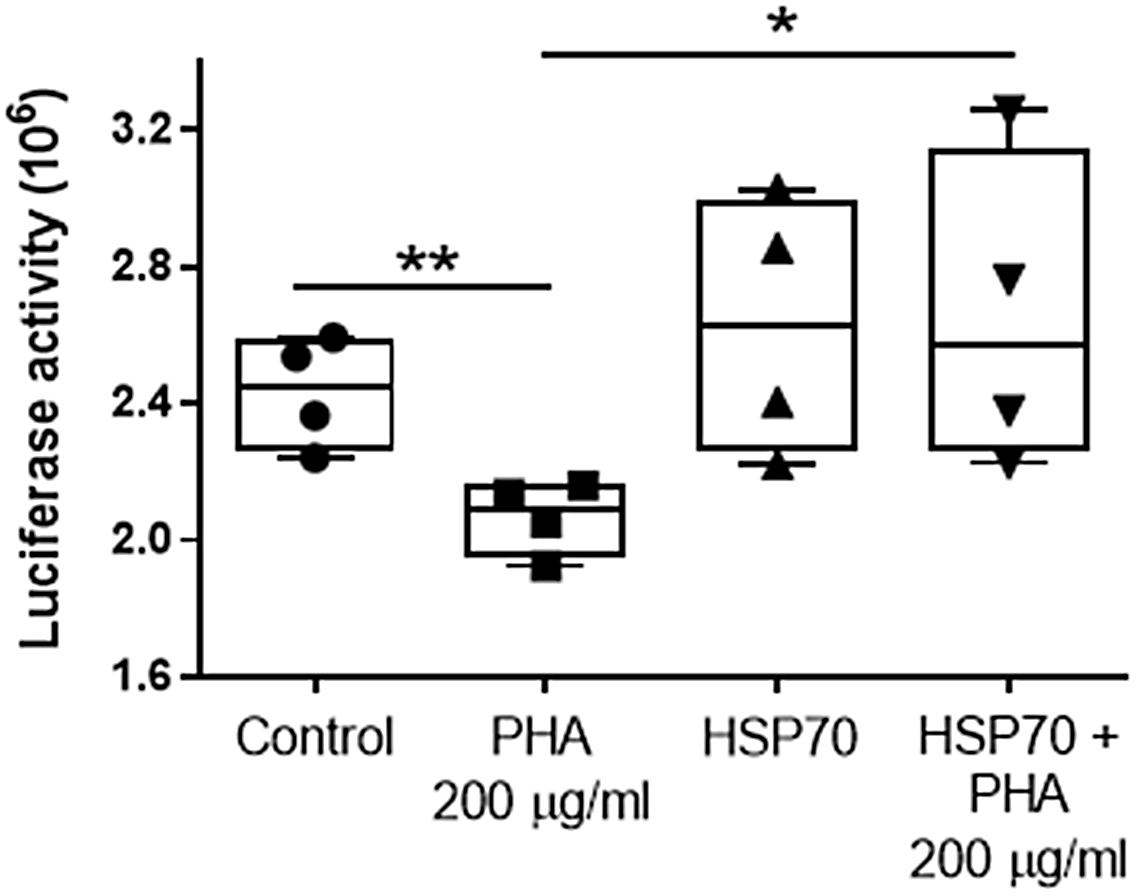
Fig. 4. The effect of HSP70 on lectin PHA (200 µg/ml for 48 h)-induced protein folding by measuring the denaturation of luciferase in Caco-2 cells (decreased luciferase activity indicates greater protein misfolding). Values are means ± SD (n = 4). * P < 0.05; ** P < 0.01.
PHA-containing raw red kidney beans decreased the expression of HSP70 and HSF1 in the small intestine
To study the effect of raw red kidney beans containing lectins in native form, qPCR analysis was performed to calculate the expression levels of HSP70 and HSF1 genes in the small intestine and colon of rats fed with RRKB. A statistically significant decreased expression of HSP70 was observed in the small intestine of RRKB-fed rats compared to control rats (relative CT values: control; 1.0 ± 1.03, RRKB; 0.34 ± 0.04, P < 0.05) (Fig. 5A). However, no difference was seen in the colon (data not shown). Following these results, we then looked at the expression levels of the HSF1 gene in the small intestine and colon. A decreased level of HSF1 was observed in the small intestine of RRKB-fed rats (relative CT values: control; 1.0 ± 0.94, RRKB; 0.13 ± 0.11, P < 0.05) (Fig. 5B), but there was no difference in the colon (data not shown).
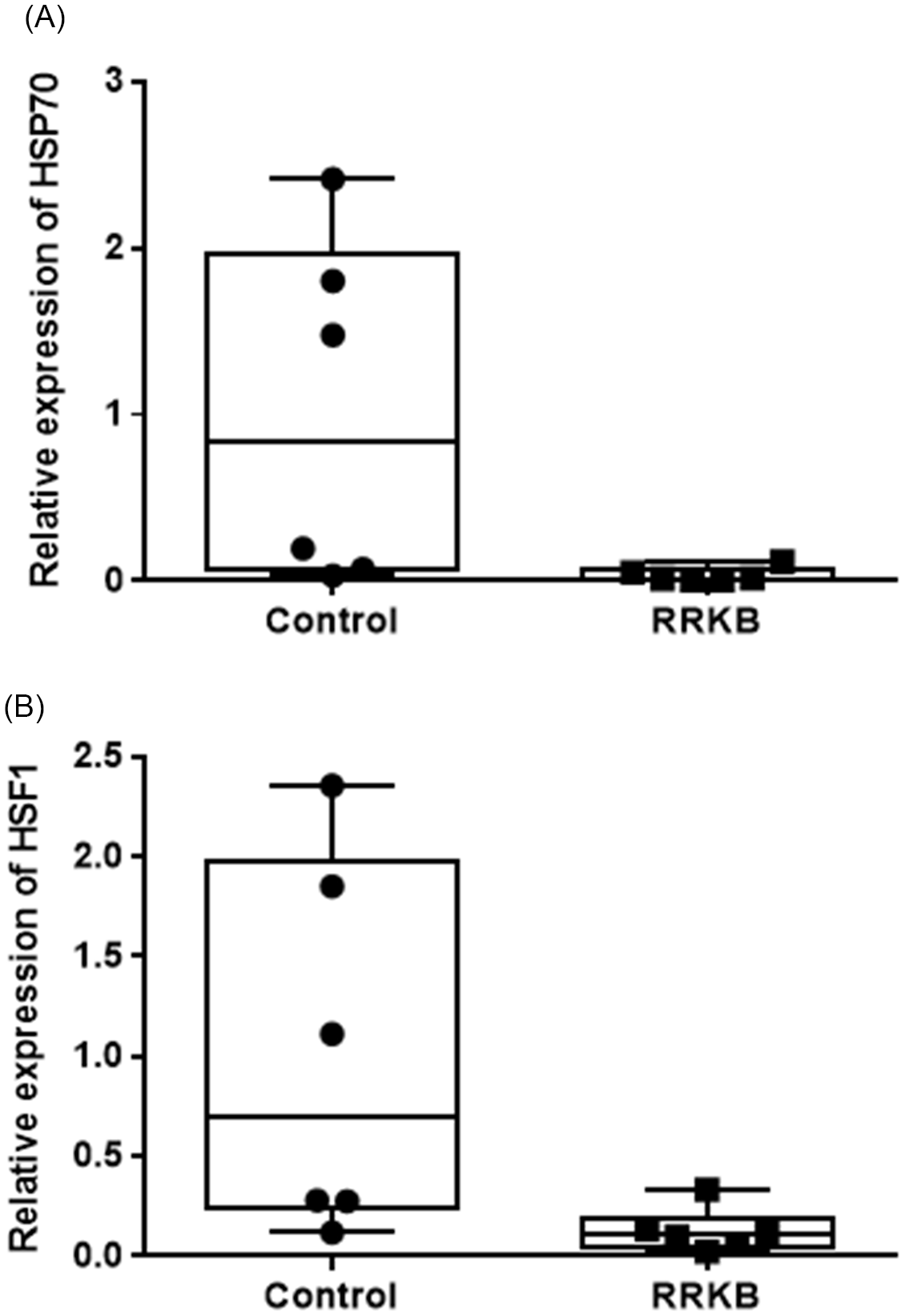
Fig. 5. Effect of raw red kidney beans (RRKB) on HSP70 and HSF1 gene expression in the small intestine of rats. qPCR analysis was performed to calculate the expression levels of HSP70 (A) and HSF1 (B) genes in the small intestine of rats fed with RRKB. A statistically significant decreased expression of HSP70 was observed in the small intestine of RRKB-fed rats compared to control rats (P < 0.05) (Figure 5A). Similarly, a reduced level (P < 0.05) of HSF1 was observed in the small intestines of RRKB-fed rats. Relative values (2–ΔΔCt) are means ± SD. n = 6 per group.
Discussion
In this study, using Caco-2 cells, we demonstrate that PHA quickly enters gut epithelial cells, decreases Caco-2 epithelial barrier function, and reduces HSP and HSF1 (heat shock factor 1) levels in the small intestine. This reduced epithelial barrier function is associated with decreased HSP70 protein expression and impaired protein folding. Lectin PHA reduces HSP70 chaperone folding efficiency in Caco-2 cells. Heat-denatured lectin PHA did not affect the epithelial barrier function. The overexpression of HSP70 reverses the folding defect, suggesting that lectin-related disturbances in protein folding, which are important in lectin toxicity, are related to impaired cellular HSP70 accumulation and function. Taken together, these results implicate the lectin-induced impairment of cellular chaperone function in disrupting epithelial barrier function.
Lectins are unique carbohydrate-binding proteins that are found in many plants and are present in high concentrations in beans and other legumes.(Reference Boylan and Sussex33,Reference Rudiger and Gabius34) Phytohemagglutinin (PHA) is a lectin in the red kidney bean (Phaseolus vulgaris). Having a carbohydrate recognition domain, PHA binds to sugars expressed on the surface of epithelial cells and exerts cytotoxicity.(Reference Sharon and Lis35) The extremely toxic effects of ricin derived from castor beans (a lethal effect at a dose as small as 500 micrograms when this lectin is inhaled by humans; http://www.bt.cdc.gov/agent/ricin/facts.asp) are related to its ability to the cell membrane, internalisation into polarised epithelial cells by specific binding to yet unknown receptors, delivery to the trans-Golgi network in the cytosol, lysosomes, or nucleus, and subsequent inhibition of protein synthesis.(Reference Spooner, Hart and Cook5,Reference Gonatas and Avrameas36–Reference Lehr38) Lectins, in contrast to mucoadhesive polymers, specifically recognise receptor-like molecules on cell membranes and bind directly to the epithelial cells rather than to the mucus layer. This receptor-mediated binding may promote the active transport of larger molecules through endocytosis.(Reference Lehr38) In mouse pituitary cells, wheat germ agglutinin-horseradish peroxidase binds to the cell surface membrane, followed by endocytosis, and targets the Golgi apparatus.(Reference Balin and Broadwell39) Additionally, glycoproteins or glycolipids can serve as lectin-binding sites.(Reference Coelho, Silva and Lima40) In this study, we found that phytohemagglutinin entered the cell rapidly to cluster around the nucleus, and the effect increased in a time-dependent manner (Fig. 2). We also found that phytohemagglutinin reduced transepithelial electrical resistance in Caco-2 cells (Fig. 1A), which was prevented when cells were exposed to heat-denatured lectin (Fig. 1B). In our previous studies, this adverse effect on transepithelial electrical resistance persisted with increased duration of exposure to 72 h, and the transepithelial electrical resistance had failed to recover to the pre-treatment levels even after washing off PHA.(Reference Ramadass, Dokladny, Moseley, Patel and Lin4)
Lectins are highly resistant to enzymatic proteolysis in the intestinal lumen and are capable of surviving heat treatment.(Reference Freed41–Reference Zakharov, Carchilan, Stepurina, Rotari, Wilson and Vaintraub44) The complete degradation of legume lectins may require severe cooking methods, such as boiling beans using a pressure cooker. Acute gastroenteritis is a well-recognised, short-term consequence of limited legume lectin exposure.(Reference Freed41,Reference Pusztai, Clarke and King45) This acute toxicity is thought to be the result of lectin binding to carbohydrate moieties on the gut epithelial surface, resulting in epithelial barrier dysfunction, maldigestion, and malabsorption.(Reference Ramadass, Dokladny, Moseley, Patel and Lin4,Reference Banwell, Howard, Cooper and Costerton46,Reference Kik, Rojer, Mouwen, Koninkx, van Dijk and van der Hage47) Another important feature of legume lectin-induced epithelial dysfunction is a loss of secretory control leading to diarrhoea.(Reference Banwell, Abramowsky, Weber, Howard and Boldt9,Reference Wilson, King, Clarke and Pusztai11,Reference King, Pusztai and Clarke48) In the present study, we showed that the toxicity of the red kidney bean lectin PHA includes a reduced expression of HSP70 and HSF1 and interference with chaperone-mediated stabilisation of protein folding.
Interesting recent literature suggests that lectin-mediated cytotoxicity in cancer cells is caused by the activation of autophagic pathways.(Reference Liu, Cheng, Bian and Bao49) Autophagy is subdivided into 3 main subtypes: chaperone-mediated autophagy, microautophagy, and macroautophagy.(Reference Dokladny, Myers and Moseley50–Reference Kaushik and Cuervo52) In chaperone-mediated autophagy, cytosolic proteins with a characteristic pentapeptide motif (KFERQ - lysine-phenylalanine-glutamic acid-arginine-glutamine)(Reference Chiang, Terlecky, Plant and Dice53,Reference Dice54) are recognised by HSPA8/HSC70 (heat shock 70 kDa protein 8) and delivered to the lysosomes via HSC70 and cochaperones, including CHIP (HSC70-interacting protein), HSP40, and HOP (HSP70–HSP90 organising protein). After motif recognition by HSPA8 and subsequent binding to LAMP2A (lysosomal-associated membrane protein 2A), the target proteins are internalised into the lysosomes, where they are unfolded and eventually degraded. The finding that autophagy is controlled, at least in part, by HSP expression(Reference Dokladny, Zuhl and Mandell55) offers the possibility that the ability of lectins to disrupt HSP accumulation and function accounts for their observed ability to activate autophagic pathways.
The folding of newly formed proteins is facilitated by protein glycosylation in the endoplasmic reticulum.(Reference Sparvoli, Faoro, Daminati, Ceriotti and Bollini56) Inhibited glycosylation leads to protein misfolding and their clustering within the endoplasmic reticulum.(Reference Hickman, Kulczycki, Lynch and Kornfeld57) Such clustered misfolded proteins attract and bind to a variety of molecular chaperones that have lectin-like activities and modulate the production of chaperones.(Reference Kuznetsov, Chen and Nigam58,Reference Pahl and Baeuerle59) We used the term “protein misfolding” to broadly describe a variety of intracellular events that destabilise proteins by hampering or distorting their ability to achieve their native conformation through unfolding, misfolding, and aggregation.(Reference Ben-Zvi and Goloubinoff60–Reference Dobson62) To perform their function, proteins must be structurally active. Since a maximally stable protein is minimally active, proteins are intrinsically unstable. Such instability threatens the success of the initial folding of the protein to achieve its native conformational structure. Even when initial folding is successful, several physicochemical challenges, such as heat, have the effect of destabilising the native conformation of newly formed protein, leading to misfolding and intracellular protein aggregation.(Reference Rieger, Morimoto and Hatzimanikatis63) To survive, cells have evolved several protective mechanisms to promote proper protein folding and deal with proteins that have unfolded, misfolded, or aggregated, including the use of heat shock proteins. In the intestine, HSP70 plays a key role as the molecular chaperone of newly formed proteins.(Reference Bukau, Weissman and Horwich64–Reference Mayer and Bukau66) In this study, we found that overexpression of HSP70 reversed the protein folding impairment caused by lectin (Fig. 4). Greater availability of HSP70 may also facilitate the process of disaggregation of proteins.
In the present study, we have focused on HSP70 as this chaperone protein is the predominant(Reference Afrazi, Sodhi and Good67) and best-characterised member of the heat shock protein family. Specifically, HSP70 is critical for protecting cells against oxidative and heat-related injury,(Reference Musch, Ciancio, Sarge and Chang68) maintenance of epithelial barrier function,(Reference Dokladny, Wharton, Lobb, Ma and Moseley28,Reference Musch, Sugi, Straus and Chang69) in part, by regulating the expression of occludin via heat shock factor-1.(Reference Dokladny, Ye, Kennedy, Moseley and Ma70) In addition, the availability of HSP70 is a key factor in cellular protection against apoptotic challenges,(Reference Ryan, Flanagan, Moseley and Gisolfi71,Reference Mosser and Martin72) suppression of TNF-α mediated cellular injury,(Reference Van Molle, Wielockx and Mahieu73,Reference Gabai, Mabuchi, Mosser and Sherman74) inhibition of endotoxin-induced shock and death(Reference Ryan, Flanagan, Moseley and Gisolfi71) through suppression of cytokine production.(Reference Kluger, Rudolph and Soszynski75–Reference Dokladny, Lobb, Wharton, Ma and Moseley77)
In the present study, we demonstrated that the red kidney bean lectin, phytohemagglutinin (PHA), rapidly entered the cell, reduced transepithelial electrical resistance in Caco-2, and interfered with protein folding. This toxic effect can be countered with the stress-related heat shock protein HSP70. The toxicity of this legume lectin could be explained by its ability to inhibit HSF1 gene expression and, in turn, the availability of HSP70. We extended the in-vitro observations by showing that exposure to plant lectin in the form of a raw red kidney bean-supplemented diet decreased HSP70 and HSF1 gene expression in rats. Our findings support the previous reports demonstrating that plant lectins inhibit intracellular HSP70 and HSP90 in the heat stress model.(Reference Ovelgonne, Koninkx and Pusztai29)
Beans represent a unique food category containing numerous beneficial nutrients, including proteins, complex carbohydrates, as well as vitamins, and minerals. They also contain potentially toxic lectins. Due to their heat resistance and imperviousness to digestive enzymes, plants containing toxic lectins are naturally protected against foraging mammals. Legumes containing foodstuffs can be consumed but only after prolonged soaking and extensive heat treatment to inactivate those toxic components.(Reference Adamcova, Laursen and Ballin1,Reference Venter and Thiel2) Prolonged aqueous boiling of beans may be sufficient for denaturing bean lectins, however, previous reports suggest that even after extended cooking or digestion in the gut, lectins may remain immunologic and exert biologic effects.(Reference Pusztai and Grant3) As we gravitate towards natural foods, the possibility that chronic low-level exposures to toxic lectins may exert adverse effects on human health is a real concern. Lectin consumption has been openly speculated to be involved in the aetiology of many pathological conditions, including common food poisoning, rheumatoid arthritis, insulin-dependent diabetes, IgA nephropathy, and peptic ulcers.(Reference Freed41) As our previous studies in animals suggested, common sucrose can be protective against potential lectin toxicity characterised by an increase in intestinal permeability, bacterial load, and translocation.(Reference Ramadass, Dokladny, Moseley, Patel and Lin4) Our present study demonstrates that boiling lectin is sufficient to deactivate its ability to disrupt the epithelial barrier. Future studies are needed to fully understand the cellular and molecular mechanisms leading to lectin-induced diminished cellular chaperone activity and its effect on tight junction proteins to develop safe interventions against lectin-induced toxicity.
The strength of our study is that it extends our knowledge of the cellular mechanisms of legume lectin toxicity. Our data show for the first time that lectin targeting heat shock proteins interferes with cellular chaperone activity, which can lead to impaired intestinal barrier function but can be rescued by overexpression of HSP70. Our findings support the injurious role of legume lectin and the protective role of HSP70 on intestinal barrier function. However, it is not known whether the findings in Caco-2 cells apply to other intestinal cell lines. Given that the epithelial barrier function is compromised by legume lectin exposure, it would be beneficial in future studies to examine the effect of PHA lectin on tight junction protein expression (occludin and ZO-1).
Since intense cooking is required to destroy legume lectins, our preference for colourful, natural-appearing foods rather than thoroughly cooked mush may lead to greater exposure to legume lectins. Exposure to these dietary toxicants is well known to cause gastrointestinal symptoms. In this study, we extended this understanding of this toxicity by showing the action of dietary lectins on protein expression and folding.
Data availability statement
Interested parties can contact Henry C. Lin, M.D. (helin@salud.unm.edu) or Karol Dokladny, Ph.D. (kdokladny@salud.unm.edu) to request access to the data.
Author contributions
K.D., P.S., P.L.M., and H.C.L. conceived and designed research; K.D. and P.S. performed experiments; K.D., P.S., and H.C.L. analysed data; K.D., P.S., P.L.M., and H.C.L. interpreted results of experiments; K.D., P.S., and H.C.L. prepared figures; H.C.L. drafted the manuscript; K.D., P.S., P.L.M., and H.C.L. edited and revised the manuscript; K.D., P.S., P.L.M., and H.C.L. approved the final version of the manuscript.
Financial support
This study was supported, in part, by the Winkler Bacterial Overgrowth Research Fund. PLM is supported by NNF14CC0001 and NNF17OC0027594.
Competing interests
The authors have no conflicts of interest to declare.
Ethical statement
This study was approved by the Institutional Animal Care and Use Committee of the New Mexico Veteran Affairs Health Care System, Albuquerque, NM. In the present studies, all methods were performed in accordance with the relevant guidelines and regulations. We also confirm that the study is reported in accordance with ARRIVE guidelines.







