Ulcerative colitis (UC) is an inflammatory bowel disease (IBD) with a chronic relapsing course, affecting the colon mucosa to a variable extent and resulting in bloody diarrhoea( Reference Meier and Sturm 1 ). Dextran sulfate sodium (DSS)-induced colitis has been used as an experimental model for UC, being clinically and histomorphologically reminiscent of the human disease. Dietary n-3 PUFA in experimental rodent models of IBD, including DSS colitis, have shown beneficial effects( Reference Camuesco, Galvez and Nieto 2 , Reference Barros, Xavier and Abreu 3 ). However, data concerning the anti-inflammatory effect of marine PUFA supplements in patients with UC are inconsistent( Reference Stenson, Cort and Rodgers 4 – Reference Almallah, Richardson and O'Hanrahan 6 ). It is therefore of interest to investigate whether dietary supplements other than fish oil (FO) can have beneficial effects on intestinal inflammation. FO may have anti-inflammatory effects through several mechanisms, including an alteration of the substrate from 20 : 4n-6 (arachidonic acid) to 20 : 5n-3 (EPA) and 22 : 6n-3 (DHA) in cell membrane phospholipids. This results in eicosanoids that have lower inflammatory potential, such as PGE3 and leukotriene B5 ( Reference Calder 7 ). Intake of FO reduced mononuclear cell production of pro-inflammatory cytokines such as IL-1β and TNFα after endotoxin stimulation( Reference Endres, Ghorbani and Kelley 8 ).
Fish peptides (FP) seem to have tissue reparative properties based on several small studies in animals and human subjects. A commercially available fish hydrolysate, containing 75–80 % protein constituents, reduced non-steroidal anti-inflammatory drug (NSAID)-induced mucosal injury in small intestine in mice( Reference Marchbank, Elia and Playford 9 ). NSAID-induced damage in small intestine and dyspeptic symptoms decreased in human volunteers( Reference Marchbank, Limdi and Mahmood 10 ). Furthermore, a dietary supplement containing bioactive peptides and amino acids seemed to beneficially affect symptoms and intestinal permeability in IBD patients( Reference Thomas, Nichols and Angstadt 11 ).
In a previous study, we found that regular intake of Atlantic salmon for 8 weeks in mildly active UC patients reduced the symptoms of disease activity and improved the fatty acid profile in plasma as well as in colon samples( Reference Grimstad, Berge and Bohov 12 ). Fish fillets may contain bioactive proteins and peptides in addition to marine fatty acids. Oxidative stress, caused by an imbalance between the formation of reactive oxygen species and counteracting antioxidants occurs in several chronic inflammatory conditions, including IBD( Reference Rezaie, Parker and Abdollahi 13 ). Increasing the antioxidant levels might reduce tissue damage and the inflammatory process. Fish and fish protein may have such an antioxidant potential. A peptide fraction from fish hydrolysate seemed to have an antioxidant effect compared with the natural antioxidant α-tocopherol( Reference Je, Park and Kim 14 ). In obese subjects consuming cod, the concentration of malondialdehyde, a measure of lipid oxidation, was reduced in blood( Reference Parra, Bandarra and Kiely 15 ).
The aim of this study was to evaluate possible anti-inflammatory effects of dietary hydrolysed salmon peptides, also named fish peptides (FP), in DSS-induced colitis in rats. Furthermore, our aim was to compare dietary intervention with FP to that of FO or combined FO + FP and assess the impact on disease activity, selected markers of inflammation and protein oxidative damage in colon tissue samples.
Experimental methods
Animals and husbandry
A total of fifty male Wistar rats (Taconic), 12 weeks old, with a weight of approximately 360 g at arrival, were placed in Makrolon III cages in an open system, with five animals in each cage. They were kept under standard laboratory conditions with 12 h dark–12 h light cycles and temperature 22 ± 1°C, relative humidity 55 ± 5 % and twenty air changes per h. The rats had free access to food and tap water. The experiment was conducted according to the Guidelines for Care and Use of Experimental Animals, and the protocol was approved by the Norwegian State Board of Biological Experiments with Living Animals.
Induction of colitis
DSS (MW 44000; TdB Consultancy AB) at 50 g/l was administered in drinking water as previously described( Reference Stucchi, Shofer and Leeman 16 ) for 7 d to induce colitis.
Experimental protocol
The rats were acclimatised for 7 d and then separated into five groups of ten rats. The following diets were given: (1) control, (2) DSS + control, (3) DSS + FO (5 %), (4) DSS + FP (3·5 %) and (5) DSS + FO + FP. All groups had free access to food on days 1–29, and DSS was administered during the last week of the experiment in groups 2–5. All rats were killed the day after DSS treatment was discontinued – on day 30. At that time the animals were anaesthetised by inhalation of Isoflurane (2 %; ‘Isoba vet’, Intervet/Schering-Plough Animal Health) in an anaesthesia chamber. Thoracotomy, cardiac puncture and exsanguination were then performed.
Diet composition
The control diet consisted of a standard casein diet with 20 % (w/w) protein (Sigma-Aldrich), and 7 % fat, composed of 5 % lard (a generous gift from Ten Kate Vetten BV) and 2 % soyabean oil (Dyets Inc.). Lard was replaced by 5 % FO in group 3 (EPAX 4020 TG, EPAX AS), and casein partly by 3·5 % FP hydrolysate (Marine BioProducts AS) in group 4, and both in group 5. The FP were produced from salmon by-products (spine) by enzymatic hydrolysis using controlled autolysis with commercial proteases. The resulting hydrolysate was further fractionated using different forms of filtration including ultrafiltration. The sizes of the peptides were <10 000 Da, with 70 % of total peptides <1000 Da. The final product consisted of >90 % peptides. The other constituents of the diets were maize starch, dyetrose, sucrose, fibre, American Institute of Nutrition (AIN)-93G mineral mix, AIN-93 vitamin mix, l-cysteine, choline bitartrate (Dyets Inc.), and tert-butyl-hydroquinone (Sigma-Aldrich) (Table 1).
Table 1. Composition of the experimental diets (g/kg diet)*

FO, fish oil; FP, fish peptides; AIN, American Institute of Nutrition.
* The diets were isonitrogenous and isoenergetic.
Weight, food intake and disease activity
Body weight and food intake were measured before DSS administration (day 23) and daily during DSS intake days 24–29. The disease activity index (DAI) is a composite score where weight loss, stool consistency and rectal bleeding are rated from 0 to 4, and the total sum is divided by 3( Reference Grimstad, Bjorndal and Cacabelos 17 ). DAI was assessed on the last day of colitis induction (day 30).
Fatty acid composition of the diets
The fatty acids were measured as previously described( Reference Grimstad, Bjorndal and Cacabelos 17 ).
Sample collection
After killing, the major part of the colon, from the colocaecal junction to the anal verge was removed, and the length was measured unstretched. The colon was rinsed in PBS, and samples from both the ends and the middle part were separated, then divided longitudinally and placed in 10 % paraformaldehyde. The samples were later embedded in paraffin and sectioned before staining with haematoxylin and eosin for histopathological examination. The remaining colon was divided into one distal and proximal segment. Pieces from both segments were cooled in liquid N2, freeze clamped and then stored at −80°C for further analyses.
Further analyses
Colon specimen
In the haematoxylin and eosin-stained tissue samples, the crypt and inflammatory scores were determined according to a validated scoring system( Reference Carrier, Aghdassi and Platt 18 ) and examined by a pathologist unaware of the experimental protocol. Crypt injury and inflammation severity scores were assessed as described earlier( Reference Grimstad, Bjorndal and Cacabelos 17 ). Both the crypt and inflammatory score include a measure of involvement: grade 1, 1–25 %; grade 2, 26–50 %; grade 3, 51–75 %; grade 4, 76–100 %. The score is the product of either the crypt grade by involvement grade or inflammation grade by the involvement grade. The histological combined score (HCS) was the sum of the crypt and inflammation scores, and was measured in all three locations of the colon.
Preparations for tissue cytokine measurements
Full wall sections of the colon were used, and 50 mg samples were homogenised and prepared as described previously( Reference Grimstad, Bjorndal and Cacabelos 17 ).
Tissue cytokine analyses
Custom-made rat cytokine four-plex kits including IL-1β, IL-5, interferon-γ and keratinocyte chemoattractant (KC)/growth-regulated oncogene (GRO) and single-plex kits with TNFα (Meso Scale Discovery) were used to measure cytokines in the supernatant from the homogenisate. The analyses were performed according to the manufacturer's recommendations. Results are given as μg/kg (wet tissue).
PG
Chemicals, solutions and sample preparation of frozen colon tissue samples (12–35 mg) were performed as described( Reference Grimstad, Bjorndal and Cacabelos 17 ).
LC/MS/MS
The LC system was an Agilent 1200 Series with a binary pump, a variable volume injector and a thermostated autosampler. HPLC separation was conducted as described( Reference Grimstad, Bjorndal and Cacabelos 17 ). Multiple reaction monitoring for data acquisition and negative ion detection was used. MassHunter software (Agilent) was used for HPLC system control, data acquisition and data processing. The PG analysed are expressed as μg/kg (wet tissue).
Markers of oxidative stress
Glutamic semialdehyde is a carbonyl product that results from direct oxidative damage of amino acids (arginyl and prolyl residues). Carboxyethyl-lysine is derived from glycosylation reactions on proteins and carboxymethyl-lysine from both glycoxidation and lipoxidation reactions. Glutamic semialdehyde, carboxymethyl-lysine and carboxyethyl-lysine concentrations were determined as trifluoroacetic acid methyl esters derivates in acid-hydrolysed, delipidated and reduced intestine protein samples. Homogenisates were measured by GC/MS as previously described( Reference Pamplona, Portero-Otin and Requena 19 ). Protein samples were prepared, and internal standards were added as described( Reference Grimstad, Bjorndal and Cacabelos 17 , Reference Pamplona, Portero-Otin and Requena 19 ). GC/MS analyses were carried out as previously described( Reference Grimstad, Bjorndal and Cacabelos 17 ). Quantification was performed by external standardisation using standard curves constructed from mixtures of 2H-labelled and non-2H-labelled standards. Analytes were detected by selected ion-monitoring GC/MS( Reference Grimstad, Bjorndal and Cacabelos 17 ). The amounts of products were expressed as the ratio of micromol per mol lysine.
Gene expression analysis
Total cellular RNA was purified from 20 mg frozen tissue samples from distal colon, RNA was quantified spectrophotometrically, and the quality was evaluated as described( Reference Grimstad, Bjorndal and Cacabelos 17 ). For each sample, 4 µg total RNA was reversely transcribed in 100 µl reactions as previously reported( Reference Grimstad, Bjorndal and Cacabelos 17 ). Real-time PCR was performed with custom-made 384-well microfluidic plates (Taq-Man Low Density Arrays; Applied Biosystems) on the following genes: Il-1b, Rn00580432_m1; Il-6, Rn 00561420; KC-GRO (KC/GRO, Cxcl1), Rn00578225_m1; inducible nitric oxide synthase 2 (Nos2), Rn00561646_m1; PPARγ (Pparg), Rn00440945; PPARγ cofactor 1α (Ppargc-1a), Rn00580241; Sirtuin 1 (Sirt1), Rn01428093_m1; PGD2 synthase (Ptgds), Rn00564605_m1; PGE synthase (Ptges), Rn00572047_m1; PG-endoperoxide synthase 2 (Cox-2, Ptgs2), Rn01483828_m1.
All probes and primers were obtained from Applied Biosystems. Two different control genes were included: 18s (Kit-FAM-TAMRA (reference RT-CKFT-18s)) from Eurogentec; and Rplp0 (Rn00821065_g1) from Applied Biosystems. To normalise the absolute quantification according to the reference genes, a second set of PCR was performed for all experimental samples, and the relative abundance values were calculated for the reference genes as well as for the target genes using standard curves derived from Universal Rat Reference RNA (Agilent Technologies Inc.). NormFinder( Reference Andersen, Jensen and Orntoft 20 ) software was used to evaluate the reference genes, and the relative abundance value of each gene in each sample was normalised against the value of the most stable reference gene, Rplp0.
Statistics
The Shapiro-Wilk test was used to test for normality of data. The Student's t test or one-way ANOVA was used to analyse differences between two or more groups, respectively. The Mann–Whitney rank test or the Kruskal–Wallis test was applied in not normally distributed data for two or more groups. Post hoc tests were performed by the Mann–Whitney rank test. A total of four pre-determined pairwise differences were analysed: FP v. DSS, FO v. DSS, FP v. FO and FO v. FO + FP. Bonferroni corrections were made in the case of multiple group analyses.
For all tests a P value ≤0·05 was considered as statistically significant. SPSS 15.0 and PASW Statistics 18 statistical software packages were used for analyses (SPSS Inc.). Bonferroni corrections were not performed in the gene expression analyses.
Results
Fatty acid composition in the diets
As intended, the FO and FO + FP diets contained large relative amounts of EPA and DHA, as well as a high total level of n-3 PUFA. The FP diet had low levels of n-3 PUFA, similar to the control diet. In the FO-containing diets, we observed a relative decrease in levels of 18 : 1n-9 as the n-3 PUFA levels increased (Table 2).
Table 2. Fatty acid compositions of the diets*
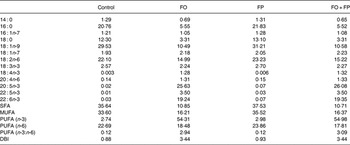
FO, fish oil; FP, fish peptides; DBI, double bond index.
* Values are represented as % (w/w) of total fatty acids.
Weight gain, feed intake and dextran sulfate sodium intake
During DSS intake all animal groups lost weight significantly compared with the control group, which in contrast gained weight. Feed intake was similar in the DSS-exposed groups, while the controls had higher food consumption (Table 3). Of the animals in the FO + FP group, two died during the DSS week.
Table 3. Average weight gain (%) and feed intake per Wistar rat (g/d) during the dextran sodium sulfate (DSS) week†
(Mean values with their standard errors for nine or ten animals per group)

FO, fish oil; FP, fish peptides.
* Mean value was significantly different from that of the control group (P ≤ 0·05; one-way ANOVA, performed only on the weight results).
† Diets were FO, FP or a combination (FO + FP) for 4 weeks, with DSS exposure in the last week of the experiment.
Histological combined score
When dividing all DSS-exposed rats into groups according to the proximal, middle or distal colon segment, there was a significant difference in HCS between the three segments (P = 0·001). Post hoc analyses showed a significantly higher HCS in the distal and middle colon segments v. the proximal segment (P = 0·002 for both differences). In the distal colon, the DSS group had a significantly higher HCS than controls( Reference Grimstad, Bjorndal and Cacabelos 17 ). There was an almost significant difference between DSS-exposed groups (P = 0·07). Post hoc analyses showed a reduced HCS after the FP diet (median 13·5; range 8–21) v. the FO diet (median 19; range 15–28) (P = 0·03). Neither FO nor FP reduced HCS v. DSS (median 17·5; range 1–20) (P = 1·0 for both differences). HCS did not differ significantly between FO and FO + FP (median 15; range 4–21) (P = 0·08) (Table 4). Histology sections illustrating the median HCS from all study groups are shown in Fig. 1.

Fig. 1. Micrograph panels. (a) Normal colonic mucosa from a healthy control rat. (b) Colonic mucosa from a rat with dextran sulfate sodium (DSS) colitis showing crypt destruction and moderate inflammation, with almost intact epithelium. (c) Colonic mucosa from a rat after DSS and fish oil (FO) diet showing crypt destruction, severe inflammation and ulcerated surface. (d) Colonic mucosa from a rat after DSS and fish peptides (FP) diet showing some preserved normal crypts and some crypt destruction, moderate inflammation and intact epithelium. (e) Colonic mucosa from a rat after FO + FP diet showing destruction of most crypts, moderate inflammation and lost epithelium. Haematoxylin and eosin staining, magnification, ×400.
Table 4. Histological combined scores (HCS) and disease activity index (DAI) in distal colon sections of Wistar rats fed fish oil (FO), fish peptides (FP) or a combination (FO + FP) for 4 weeks, and treated with dextran sodium sulfate (DSS) during the last week of the experiment
(Median values and ranges)

* Post hoc tests analysed by the Mann–Whitney rank test.
Disease activity index
The median DAI value in the DSS group was higher than in the control group( Reference Grimstad, Bjorndal and Cacabelos 17 ). DAI differed significantly between DSS-exposed groups (P = 0·02). Post hoc tests showed no significant reduction of DAI in the FP group (median 1·3; range 0·7–3·7) v. the FO group (median 2·8; range 1·3 – 3·7) (P = 0·07). DAI was higher after the FO diet than after DSS alone (median 1·0; range 0·3 – 3·0) (P = 0·04). DAI did not differ between FP and DSS (P = 1·0), and did not differ in the FO v. the FO + FP group (median 1·3 range 0·7 – 3·7) (P = 0·6) (Table 4).
Colon length
The controls had a significantly longer colon than the DSS group, reflecting a colitis-induced shortening after DSS exposure( Reference Grimstad, Bjorndal and Cacabelos 17 ). There were no differences in colon length between the DSS-exposed groups (P = 0·20), but there was a tendency to have a longer colon in the FP group (data not shown).
PG
PGE3 levels were significantly different between the DSS-exposed groups in the distal colon (P = 0·001). Post hoc analyses showed that the FO diet increased PGE3 levels v. DSS alone. PGE3 levels were not significantly different in the FP v. the DSS group. The combined FO + FP group gave an additional increase of PGE3 as compared with the FO group (Table 5). In the case of PGE2, the difference between DSS groups was significant (P = 0·04) in the distal colon. However, none of the preselected post hoc tests revealed any differences. The largest difference in PGE2 levels occurred between the DSS and the FO + FP group. For PGD2, a significant difference (P = 0·04), was detected between DSS groups. In the distal colon, the FO + FP diet increased PGD2 compared with FO (Table 5).
Table 5. Selected gene expression, and cytokine and prostaglandin levels in distal colon sections of Wistar rats fed fish oil (FO), fish peptides (FP) or a combination (FO + FP) for 4 weeks, and treated with dextran sodium sulfate (DSS) during the last week of the experiment†
(Median values and ranges)

Nos2, inducible nitric oxide synthase 2; KC, keratinocyte chemoattractant; GRO, growth-regulated oncogene.
a,b,c Median values for the DSS groups with unlike superscript letters were significantly different (P < 0·05 (Kruskal–Wallis test/Mann–Whitney post hoc test) except KC/GRO: P = 0·07 (Kruskall–Wallis test)).
** Median value was significantly different from that of the control treatment (P < 0·01; Mann–Whitney rank test).
† Nos2 expression values were normalised against an endogenous control, and variables are given as medians relative to control (n 7–8). Cytokine and prostaglandin levels (μg/kg wet tissue) are given as medians (n 8–10).
Gene expression
The expression of mRNA was significantly increased after DSS alone v. control for several selected genes, including Il-6, Il-1b, Cxcl1 and Ptges ( Reference Grimstad, Bjorndal and Cacabelos 17 ). The FP diet reduced the mRNA levels of these genes compared with DSS alone, although not reaching significant levels. Nos2 expression was significantly different between the DSS-exposed groups (P < 0·05). FP, FO and FO + FP diets increased Nos2 mRNA levels v. DSS, although only significantly for the FO + FP diet (Table 5).
Cytokines
The protein levels of TNFα, IL-1β and KC/GRO in the distal colon were in accord with mRNA levels. All three cytokines were significantly higher in the DSS animals v. the controls( Reference Grimstad, Bjorndal and Cacabelos 17 ). KC/GRO was close to significantly different between the DSS-exposed groups (P = 0·07). Post hoc tests revealed that the FP diet significantly lowered the KC/GRO levels v. the FO diet. The other preselected pairs analysed showed no significant differences. No significant differences in TNFα or IL-β occurred between groups that received DSS.
Markers of oxidative stress
No significant differences were detected between the DSS-exposed groups or between selected pairs for the three markers (data not shown). The levels were higher in the DSS v. the controls for all three markers although not reaching significance. We noted that, compared with DSS, median levels of all three markers tended to decrease after FO, while carboxyethyl-lysine and carboxymethyl-lysine levels were lower after FP, although not significantly.
Discussion
The main findings of this study are that hydrolysed salmon peptides, a novel dietary component used in IBD models, attenuated DSS-induced colitis as compared with FO. This was observed by fewer signs of histopathological inflammation, less clinical disease activity, a lowered inflammatory cytokine signature at the protein and mRNA levels, and increased PGD2. Also, the FP diet improved a number of variables when compared with animals fed DSS alone, such as cytokines and markers of protein oxidation, although not reaching statistical difference. In addition, we observed that the expected increase of PGE3 induced through metabolism of EPA from dietary FO increased further after combining FO and FP in the diet.
Although intake of FP has not been much investigated in experimental colitis, other dietary bioactive peptides have shown anti-inflammatory effects in IBD animal models( Reference de Medina, Daddaoua and Requena 21 ). An intestinal protective effect from fish hydrolysate was suggested, as proliferation of HT 29 cells increased, while indomethacin-induced apoptose and small-intestinal villous damage were decreased in mice( Reference Marchbank, Elia and Playford 9 ). Moreover, intake of fish protein hydrolysate containing 75 – 80 % protein constituents in healthy humans reduced small-intestinal damage caused by indomethacin( Reference Marchbank, Limdi and Mahmood 10 ). The proposed mechanisms in these two studies were the presence of glutamine (14 – 16 % of the total), an antioxidant effect, and an effect of the 6 – 10 % content of FO in the hydrolysate. The doses of 25 – 50 g/l and 3 g daily used in their studies are not directly comparable with 35 g/kg used in our study. FP contained > 90 % peptides, but had a lower glutamine content of 8·5 % (w/w). Glutamine, a substrate for enterocyte metabolism, could act protectively by preserving intestinal integrity and barrier function, as shown in previous experimental colitis models( Reference Coeffier, Marion-Letellier and Dechelotte 22 ). The n-3 content in FP was only 3·0 %, similar to the controls in our study (2·7 %), and can probably not explain the beneficial changes after the FP diet. Specific structural features have been linked to potentially bioactive peptides; the molecular weight should be <10 kDa, most preferably <1 kDa, and the size of peptides between two and twenty amino acids( Reference Ryan, Ross and Bolton 23 ). Our FP product is within this range.
DSS-induced experimental colitis has some similarities to the human disease UC. Epithelial injury is fundamental in this model, and during the acute colitis phase innate immune responses are activated( Reference Hall, Faivre and Quinlan 24 ). The pro-inflammatory cytokine levels in the DSS model were at their lowest after the FP diet at both the protein and mRNA levels, suggesting an anti-inflammatory effect from bioactive FP, influencing innate immunity. PGD2 reduces granulocyte infiltration in experimental colitis( Reference Ajuebor, Singh and Wallace 25 ), and its synthesis is up-regulated in UC patients in remission( Reference Vong, Ferraz and Panaccione 26 ). The tendency for PGD2 to increase after FP diets in our study may point to a protective effect.
Contrary to expectations, FO did not improve signs of colitis. As compared to DSS, HCS was unaltered and DAI increased. Other variables were not significantly altered. This is in contrast to previous trials with dietary n-3 PUFA administration in experimental colitis, which has generally shown beneficial effects( Reference Vilaseca, Salas and Guarner 27 – Reference Nieto, Torres and Rios 29 ). The fatty acid analysis of the diets confirmed large relative amounts of EPA and DHA in the two FO diets, with an n-3:n-6 ratio in the feed of 2·9 and 3·1. The relatively anti-inflammatory eicosanoid PGE3 was increased, which is expected after dietary intake of EPA. In our study however, vitamin E or other anti-oxidants were not added to the diets containing FO. This could result in increased lipid peroxidation and oxidative stress in the colonic mucosa, which could counteract an anti-inflammatory effect( Reference Meydani 30 ). However, the levels of oxidation markers in our study were not increased, but rather tended to decrease, after the FO diet.
When combining FO and FP diets we observed that the PGE3 levels increased as compared to the FO diet. The reason for this is unclear, as adding the FP diet did not provide high amounts of EPA. FP, when combined with the FO diet, could possibly influence the enzymatic activity of cox2, thus leading to elevated PGE3 synthesis. We observed that the mRNA levels of ptgs2/cox2 as well as ptges were elevated in the combined FO + FP group v. FO, but not reaching significance.
Nos2 expression was elevated after DSS v. controls, and was significantly increased after the FO + FP diet v. DSS alone. NO is a result of inducible NO synthase expression, and seems to have a dual role. It may provide either anti-inflammatory or toxic effects( Reference Cross and Wilson 31 ). Thus, the higher Nos2 levels after the FO + FP diet may be a protective response, supported by the PGE3 and PGD2 increase, or it may be detrimental, as suggested by a tendency for elevated HCS and cytokine levels.
This study has its limitations: antioxidant added to the FO diets might have influenced the results. An increased number of rats in each group would have increased the statistical power of the analyses. Statistical analysis regarding feed and DSS intake could not be performed. We induced a severe colitis, leading to death in two animals, by administering 5 % DSS for 1 week, which could limit the detection of modest beneficial effects. The diets used might affect the healing time of the colitis after stopping DSS. The effects observed may be due to prophylactic as well as therapeutic potential of the diets, as the study design cannot differentiate between these aspects. The DSS model clearly has limitations as a substitute for the human disease, as a chemically induced injury model instead of an endogenously triggered inflammation.
In conclusion, dietary hydrolysed salmon peptides may offer protection against DSS colitis as compared with a FO diet, but do not improve inflammatory variables significantly when compared with DSS alone. A combined diet may affect eicosanoid metabolism by increasing PGE3 levels, although not attenuating other signs of colon inflammation. Hydrolysed salmon peptides should be further investigated as a dietary intervention against inflammation in animal models, as well as in patients with IBD.
Acknowledgements
Ingeborg Kvivik, Kari Espedal and Marita Hanasand are thanked for their efforts during the cytokine analyses and Torunn Eide for the analyses of PG. We thank Eline Milde, Kari Williams and Randi Sandvik for excellent technical assistance. Jan Terje Kvaløy is thanked for valuable statistical advice.
Contributions: T. G., B. B., T. H. and R. K. B. developed the study concept and design. T. G. and B. B. conducted the animal study, analysed and interpreted the data. T. G. drafted the manuscript. D. C., M. P. O. and R. P. carried out the oxidative marker analyses. O. G. A. conducted the histological evaluation, R. O. worked with cytokine analyses and A. S. carried out the PG analyses. P. B. measured the diet fatty acids. T. G., T. H., R. O. and R. B. K. made critical revisions of the manuscript. All authors read and approved the final manuscript.
The authors have no conflict of interest.
This work was supported by the Board of Nutrition Programmes (University of Bergen, Western Norway Regional Health Authority and the Institute of Marine Research). D. C. holds a fellowship from the Spanish Ministry of Health (FI0800707). Work carried out at the Department of Experimental Medicine was supported by grants from the Spanish Ministry of Science and Innovation (BFU2009-11879/BFI), the Autonomous Government of Catalonia (2009SGR735), and the Spanish Ministry of Health (PI081843) to M. P. O. and R. P. This work was also supported by COST B-35 action.







