Introduction
Brachiopods, as important constituents of the benthic marine ecosystems, represent a useful tool to shed light on the environmental and ecological shifts across the Phanerozoic. Actually, numerous marine biotic crises and mass extinction events recurrently affected the phylum Brachiopoda, generating loss of biodiversity and affecting the evolutionary trajectories of different brachiopod clades (Gould and Calloway, Reference Gould and Calloway1980; Chen et al., Reference Chen, Kaiho and George2005; Curry and Brunton, Reference Curry, Brunton and Selden2007; García Joral et al., Reference García Joral, Gómez and Goy2011; Harper et al., Reference Harper, Hammarlund and Rasmussen2014; Carlson, Reference Carlson2016; Finnegan et al., Reference Finnegan, Rasmussen and Harper2016, Reference Finnegan, Rasmussen and Harper2017; Vörös et al., Reference Vörös, Kocsis and Pálfy2016, Reference Vörös, Kocsis and Pálfy2019; Baeza-Carratalá and García Joral, Reference Baeza-Carratalá and García Joral2020). Of these clades, the Order Rhynchonellida was affected by all the classical great extinction and diversification events, but it is still extant in the present-day marine ecosystems, becoming an effective tool for understanding the evolution and ecology of these ecosystems over time.
In addition to the long-established “Big Five” mass extinction events, Vörös et al. (Reference Vörös, Kocsis and Pálfy2019) defined several episodes of synchronous extinction of diverse brachiopod orders as clade extinctions, including, in the Early Jurassic, the Early Toarcian Mass Extinction Event (ETMEE) as the last clade extinction of brachiopods at an ordinal level. The ETMEE, which was one of the most significant environmental perturbations of the Mesozoic, represented a severe extinction for the brachiopod fauna (García Joral and Goy, Reference García Joral and Goy2000, Reference García Joral and Goy2009; Vörös, Reference Vörös2002; Comas-Rengifo et al., Reference Comas-Rengifo, García Joral and Goy2006; García Joral et al., Reference García Joral, Gómez and Goy2011, Reference García Joral, Baeza-Carratalá and Goy2018; Baeza-Carratalá et al., Reference Baeza-Carratalá, García Joral, Giannetti and Tent-Manclús2015, Reference Baeza-Carratalá, Manceñido and García Joral2016a, Reference Baeza-Carratalá, Reolid and García Joral2017; Vörös et al., Reference Vörös, Kocsis and Pálfy2016, Reference Vörös, Kocsis and Pálfy2019; Baeza-Carratalá and García Joral, Reference Baeza-Carratalá and García Joral2020). Fluctuation in brachiopod diversity dynamics can be detected early in the latest Spinatum (Pliensbachian) and in the Tenuicostatum (Toarcian) chronozones in the western Tethys, within the so-called “Extinction Interval” (ca. 182.0–184.0 Ma), culminating in a severe loss of diversity in the “Extinction boundary” (ca. 182.0–182.6 Ma) dated in the earliest Serpentinum Chronozone (e.g., García Joral et al., Reference García Joral, Gómez and Goy2011; Caruthers et al., Reference Caruthers, Smith and Gröcke2013; Baeza-Carratalá et al., Reference Baeza-Carratalá, García Joral, Giannetti and Tent-Manclús2015, Reference Baeza-Carratalá, Reolid and García Joral2017; Danise et al., Reference Danise, Clémence, Price, Murphy, Gómez and Twitchett2019; Krencker et al., Reference Krencker, Fantasia, Danisch, Martindale, Kabiri, El Ouali and Bodin2020; among many others).
Several adaptive strategies within the brachiopod fauna were suggested around this ecological crisis (García Joral et al., Reference García Joral, Gómez and Goy2011, Reference García Joral, Baeza-Carratalá and Goy2018; Baeza-Carratalá et al., Reference Baeza-Carratalá, García Joral, Giannetti and Tent-Manclús2015, Reference Baeza-Carratalá, Reolid and García Joral2017, Reference Baeza-Carratalá, García Joral, Goy and Tent-Manclús2018a; Vörös et al., Reference Vörös, Kocsis and Pálfy2016; Piazza et al., Reference Piazza, Duarte, Renaudie and Aberhan2019, Reference Piazza, Ullmann and Aberhan2020; Ullmann et al., Reference Ullmann, Boyles, Duarte, Hesselbo, Kasemanns, Kleins, Lenton, Piazza and Aberhan2020). In this sense, rhynchonellides underwent a notable renewal. Consequently, before analyzing the ecological effects and/or evolutionary implications activated around ETMEE, it is essential to clarify the taxonomy of rhynchonellide species in the pre- and post-extinction intervals.
As previous authors conveyed (e.g., Tomašových, Reference Tomašových2006), the taxonomy of Lower Jurassic multicostate rhynchonellides is far from being totally resolved. The basal stock of Jurassic ribbed rhynchonellides underwent an immediate diversification after the end-Triassic crisis in the intra-Tethyan and NW-European platforms, as early as in the Hettangian–early Sinemurian (Rossi-Ronchetti and Brena, Reference Rossi Ronchetti and Brena1953; Gaetani, Reference Gaetani1970; Alméras and Hanzo, Reference Alméras and Hanzo1991; Dulai, Reference Dulai1993, Reference Dulai2001, Reference Dulai2003; Böhm et al., Reference Böhm, Ebli, Krystin, Lobitzer, Rakús and Siblík1999; Siblík, Reference Siblík1999; Tomašových, Reference Tomašových2006, Baeza-Carratalá et al., Reference Baeza-Carratalá, Dulai and Sandoval2018b). Several representative taxa that underwent this diversification are included in the Superfamily Wellerelloidea.
Superfamily Wellerelloidea Licharew, Reference Licharew1956, is a long-range superfamily from the lower Carboniferous to the Lower Jurassic (Ager et al., Reference Ager, Childs and Pearson1972; Manceñido, Reference Manceñido2000; Manceñido and Owen, Reference Manceñido, Owen, Brunton, Cocks and Long2001; Savage et al., Reference Savage, Manceñido, Owen, Dagys, Dong-Li and Kaesler2002), with ~54 genera arranged in six families and 10 subfamilies. Among them, Subfamily Cirpinae Ager, Reference Ager and Moore1965, is a lineage of multicostate Wellerellidae mainly extending through the Upper Triassic to the lower Toarcian. Subfamily Cirpinae encompasses the last representatives of Wellerelloidea in the Pliensbachian-Toarcian transition in coincidence with a timespan where crucial ecological perturbations occurred, ending up in the ETMEE.
In the peri-Iberian platforms system, the abundant record of Cirpinae in the Early Jurassic is represented by the genera Calcirhynchia, Cirpa, and Salgirella (e.g., Pérez-López et al., Reference Pérez-López, Martín-Algarra, Alméras and Foucault1993; Alméras and Fauré, Reference Alméras and Fauré2000; Comas-Rengifo and Goy, Reference Comas-Rengifo and Goy2010; Comas Rengifo et al., Reference Comas-Rengifo, Duarte, García Joral and Goy2013, Reference Comas-Rengifo, Duarte, Félix, García Joral, Goy and Rocha2015; Baeza-Carratalá, Reference Baeza-Carratalá2013). The genus Calcirhynchia seems to be restricted to the Sinemurian–lowermost Pliensbachian deposits (Pérez-López et al., Reference Pérez-López, Martín-Algarra, Alméras and Foucault1993; Baeza-Carratalá, Reference Baeza-Carratalá2013; Baeza-Carratalá et al., Reference Baeza-Carratalá, Giannetti, Tent-Manclús and García Joral2014, Reference Baeza-Carratalá, Dulai and Sandoval2018b), while the prolific occurrence of Cirpa and Salgirella throughout the entire Early Jurassic was suddenly terminated by the onset of the ETMEE, even by the Pliensbachian-Toarcian transition. The present paper clarifies the complex systematics of the Lower Jurassic rhynchonellides, unravelling the record of the last representatives of the Superfamily Wellerelloidea worldwide. The Pliensbachian–Toarcian wellerelloids are accurately appraised, formally described, and discussed in light of new records from the Betics and Lusitanian basins. The conspicuous record of Cirpinae in these areas enables us to suggest diagnostic criteria to discriminate taxa morphometrically.
Finally, revision of the biogeographic distribution of this rhynchonellide clade in the western Tethys and its evolutionary history in the Early Jurassic has been undertaken, bearing in mind their conceivable demise in relation to the ETMEE.
Geological setting
In paleogeographical terms, the Jurassic peri-Iberian platforms system constituted, together with the North African margins, the westernmost coastline of the Tethys Ocean. In the Early Jurassic, the proto-Atlantic seaway connected this part of the Tethys with Panthalassa via the Hispanic Corridor (e.g., Manceñido, Reference Manceñido1990, Reference Manceñido2002; Manceñido and Dagys, Reference Manceñido, Dagys and Westermann1992; Damborenea, Reference Damborenea2000; Aberhan, Reference Aberhan2001; Sha, Reference Sha2002; García Joral et al., Reference García Joral, Gómez and Goy2011). Pliensbachian–Lower Toarcian wellerelloids have been reported around the Iberian Plate in the Asturian Basin (Comas-Rengifo and Goy, Reference Comas-Rengifo and Goy2010), the Betic Ranges (e.g., Baeza-Carratalá, Reference Baeza-Carratalá2011, Reference Baeza-Carratalá2013), and the Lusitanian Basin (Comas-Rengifo et al., Reference Comas-Rengifo, Duarte, García Joral and Goy2013, Reference Comas-Rengifo, Duarte, Félix, García Joral, Goy and Rocha2015). Their occurrence in the northernmost area of the Iberian Range (Rodrigo, Reference Rodrigo2011) is questionable, as later discussed. A concise description of the depositional framework follows, describing the living environment of this group.
External Subbetic Domain
In the easternmost Betic Range (Alicante and Murcia provinces, East Spain), representatives of Cirpa and Salgirella are recorded in Pliensbachian–lowermost Toarcian sediments from the epioceanic External Subbetic area (Baeza-Carratalá, Reference Baeza-Carratalá2013), which is characterized by pelagic seamount facies. Most of the taxa are recorded in the upper member of the Gavilán Formation (late Pliensbachian), which consists of red crinoidal grainstone beds with abundant glauconite, peloids, and intraclasts, with occasional calcarenite levels interspersed. Only the record of Cirpa briseis reaches up to the marly levels of the Zegrí Formation (uppermost Pliensbachian–lowermost Toarcian) (Fig. 1), which consists of thin beds of alternating yellowish and greenish marls and marly mudstone with sporadic calcarenites and yellowish sandy marlstone beds.
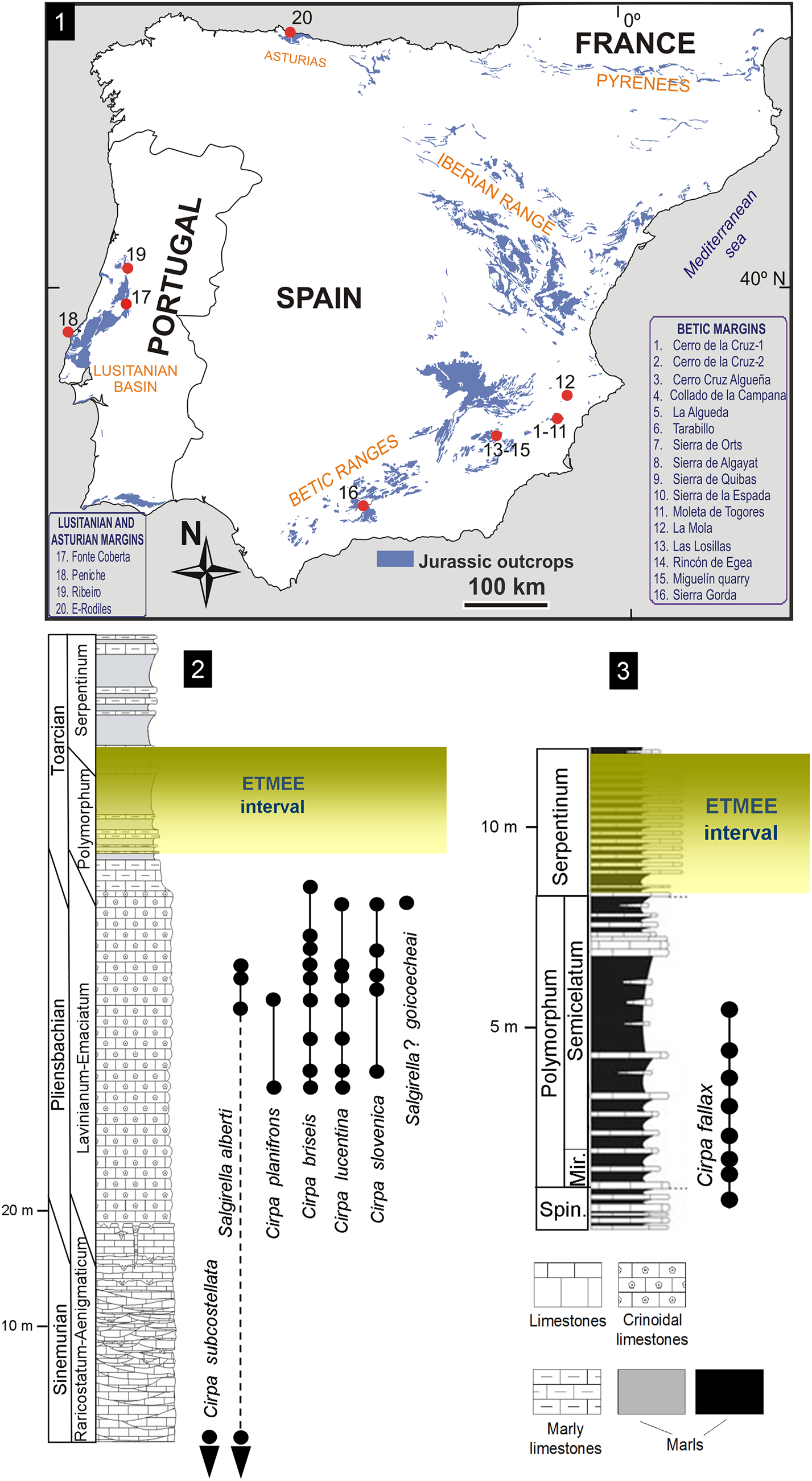
Figure 1. (1) Locations of the studied Pliensbachian–Lower Toarcian outcrops in the peri-Iberian platforms system, with wellerelloids among the constituents of the brachiopod assemblages. (2, 3) Synthetic Lower Jurassic stratigraphical sections showing the distribution of Wellerelloidea species in (2) the Betic Ranges and (3) the Lusitanian basin. Mir. = Mirabile Subzone; Spin. = Spinatum Zone.
Transitional External Betic Zones
Pliensbachian wellerelloids were also documented in the transitional areas of the Prebetic–Subbetic domains (La Mola region, Alicante, East Spain; Baeza-Carratalá et al., Reference Baeza-Carratalá, García Joral and Tent-Manclús2016b). This region is considered a linking area between shallow epicontinental platforms that prevailed in the Prebetic on the North and the Subbetic Domain characterized by pelagic seamount facies southwards. In this area, red crinoidal grainstone sediments, comparable to those of the External Subbetic area, characterize the upper Pliensbachian deposits (Baeza-Carratalá et al., Reference Baeza-Carratalá, García Joral and Tent-Manclús2016b).
Internal Subbetic Domain
This area is located in Sierra Gorda (Granada Province, South Spain). The wellerelloid-bearing deposits are represented by the intermediate and upper members of the Gavilán Formation (Sinemurian–earliest Pliensbachian in age). The intermediate member consists of a thick carbonate succession (Sandoval, Reference Sandoval1983; García-Hernández et al., Reference García-Hernández, Lupiani and Vera1986) characterized by micritic peloidal mudstone/packstone levels, sometimes with oolitic/oncolitic grainstone to packstone beds and algal wackestones containing large foraminifera, sponges, bivalves, gastropods, and echinoderms. The depositional setting was interpreted as shallow neritic platforms with occasional protected areas involving low- to middle-energy environments (Olóriz et al., Reference Olóriz, Linares, Goy, Sandoval, Caracuel, Rodríguez-Tovar, Tavera, Gibbons and Moreno2002; Ruiz-Ortiz et al., Reference Ruiz-Ortiz, Bosence, Rey, Nieto, Castro and Molina2004). Overlying these sediments, exposures of the upper member of Gavilán Formation, which are represented by crinoidal grainstone/packstone carbonates with crinoids, brachiopods, and bivalves, are interpreted as high-energy deposits in external-platform, tidal to intertidal areas, that accumulated during progressive drowning of the Early Jurassic platforms (Olóriz et al., Reference Olóriz, Linares, Goy, Sandoval, Caracuel, Rodríguez-Tovar, Tavera, Gibbons and Moreno2002).
Lusitanian Basin
Portuguese Cirpa are found mainly in Toarcian sediments from the Rabaçal-Condeixa region and the Peniche area (Lusitanian Basin). They are recorded in Rabaçal in the basal deposits of the São Gião Formation (Comas-Rengifo et al., Reference Comas-Rengifo, Duarte, García Joral and Goy2013; Piazza et al., Reference Piazza, Duarte, Renaudie and Aberhan2019) as whitish gray marly beds (Fig. 1). In Peniche, Cirpa occurs in the lower member of the Cabo Carvoeiro Formation (Alméras, Reference Alméras, Copper and Jin1996; Comas-Rengifo et al., Reference Comas-Rengifo, Duarte, Félix, García Joral, Goy and Rocha2015), which consists of predominantly bioturbated and ferruginous gray marls with a few intercalations of cm-thick marly limestones. Both formations correspond to a low-energy, distal homoclinal ramp, deeper to the west/northwest, typified by hemipelagic sequences and facies rich in organic matter, where an alternation of marlstone and argillaceous limestone beds prevailed (Duarte, Reference Duarte2007).
Asturian Basin
The only wellerelloid documented in the Asturian Basin is the pervasive Cirpa briseis (Comas-Rengifo and Goy, Reference Comas-Rengifo and Goy2010) in deposits of the Rodiles Formation (Pliensbachian, Davoei–Margaritatus chronozones). The Rodiles Formation consists of a rhythmically alternating succession of marls and micritic and marly limestone beds, typifying a depositional environment consisting in an open sea carbonate ramp (Valenzuela et al., Reference Valenzuela, García-Ramos and Suárez de Centi1986). Cirpa briseis probably reached this basin in the transgressive maximum that occurred in the early-late Pliensbachian transition (Comas-Rengifo and Goy, Reference Comas-Rengifo and Goy2010).
Materials and methods
We included 570 wellerelloids in the analysis: 363 specimens were collected and studied bed-by-bed in Lower Jurassic deposits from several localities in the Betic Ranges (Fig. 1.1), which are summarized in a synthetic stratigraphical section (Fig. 1.2). They are deposited at the Earth and Environmental Sciences Department at the University of Alicante (DCTMA). In addition, 45 specimens deposited at the Department of Geodynamics, Stratigraphy and Paleontology (Universidad Complutense de Madrid; DPUCM) were collected from the Pliensbachian-Toarcian transition (Fig. 1.3) in Fonte Coberta and Peniche (Lusitanian Basin). Taxonomic identifications were complemented by material (160 specimens) in the Jiménez de Cisneros historical collection (JdC collection hereafter) held at the Paleontological Museum of Murcia (Spain), after a critical systematic revision (Baeza-Carratalá, Reference Baeza-Carratalá2008), and two specimens from the Peiró collection deposited at the Paleontological Museum of Elche (MUPE; Alicante, Spain).
The ammonoid chronostratigraphical zonal scheme is based on the standard zones proposed by Cariou and Hantzpergue (Reference Cariou and Hantzpergue1997), Dommergues et al. (Reference Dommergues, Meister and Mouterde1997), Elmi et al. (Reference Elmi, Roulleau, Gabilly and Mouterde1997), and Page (Reference Page2003) for the Tethys Realm.
For the morphometric analysis, the main biometric parameters were measured directly on the specimens using calipers. Additional representative external biometric attributes were selected and quantitatively computed (Fig. 2 and supplementary material). The 96 specimens on which all of the 10 external biometric parameters could be measured were then used for the analysis (Fig. 2 and supplementary material). Subsequently, Principal Component Analysis (PCA) and discriminant Canonical Variate Analysis (CVA) were conducted as exploratory methods for variable reduction (Hammer and Harper, Reference Hammer and Harper2006). These analyses were carried out by means of the PAST 3.22 software package (Hammer et al., Reference Hammer, Harper and Ryan2001) using a correlation matrix for the PCA because continuous as well as discrete variables were included.

Figure 2. Main biometric parameters measured in the morphological analysis. L = length; W = width; T = thickness; hf = height of dorsal median fold; wb = basal width of dorsal median fold; wt = upper width of dorsal median fold; dpl = maximum width of the intercostal area flanking the fold; dr = tangential distance between ribs marking smooth intercostal area alongside the fold. Not shown on Figure 2: R = total number of ribs; Rf = number of ribs on the fold.
Internal structure and microstructural analyses of the shell were conducted using the conventional method of preparing oriented transverse serial sections and taking acetate peels. The distance between serial sections was 0.1 mm. High resolution photomicrographs of acetate peels were taken under an optical microscope (Nikon CFI60 E600POL). The obtained peels are deposited at DCTMA (Alicante) and DPUCM (Madrid).
Whenever possible, specimens were coated with magnesium oxide prior to photographing. Biogeographical distribution analysis was performed and plotted on a base paleomap (slightly modified after Bassoullet et al., Reference Bassoullet, Elmi, Poisson, Ricou, Cecca, Bellión, Giraud, Baudin, Decourt, Ricou and Vrielynck1993).
Repositories and institutional abbreviations
Collections of the Earth and Environmental Sciences Department at the University of Alicante, Spain (DCTMA); Paleontological Collections at Department of Geodynamics, Stratigraphy and Paleontology of the Universidad Complutense de Madrid, Spain (DPUCM); Jiménez de Cisneros historical collection (JdC) deposited at the Paleontological Museum of Murcia (Murcia, Spain); Peiró collection held at Paleontological Museum of Elche (Alicante, Spain) MUPE.
Systematic paleontology
Phylum Brachiopoda Duméril, Reference Duméril1805
Subphylum Rhynchonelliformea Williams et al., Reference Williams, Carlson, Brunton, Holmer and Popov1996
Class Rhynchonellata Williams et al., Reference Williams, Carlson, Brunton, Holmer and Popov1996
Order Rhynchonellida Kuhn, Reference Kuhn1949
Superfamily Wellerelloidea Licharew, Reference Licharew1956
Family Wellerellidae Licharew, Reference Licharew1956
Subfamily Cirpinae Ager, Reference Ager and Moore1965
Genus Cirpa De Gregorio, Reference De Gregorio1930
Type species
Rhynchonella (Cirpa) primitiva De Gregorio, Reference De Gregorio1930
Cirpa briseis (Gemmellaro, Reference Gemmellaro1874)
Figure 3.15–3.28
- Reference Gemmellaro1874
Rhynchonella briseis Gemmellaro, p. 97, pl. 11, figs. 19–22.
- Reference Haas1884
Rhynchonella briseis Gemmellaro; Haas, p. 4. pl. 1, figs. 3–5 (non fig. 6).
- Reference Parona1884
Rhynchonella briseis Gemmellaro; Parona, p. 244, pl. 3, fig. 1 (part; ? pl. 2, figs. 10–20; ? pl. 3, fig 2).
- Reference Geyer1889
Rhynchonella variabilis Schlotheim; Geyer, p. 36, pl. 4, figs. 19–21.
- Reference Di Stefano1891
Rhynchonella briseis Gemmellaro; Di Stefano, p. 208, pl. 3, figs. 9–13 (part; not var. iphimedia, pl. 3, figs. 14–17).
- Reference Parona1892
Rhynchonella briseis Gemmellaro; Parona, p. 29, pl. 2, fig. 1 (part; not pl. 2, figs. 2–7).
- Reference Böse1897
Rhynchonella briseis Gemmellaro; Böse, p. 184, pl. 13, fig. 20.
- Reference Böse and Schlosser1900
Rhynchonella variabilis Schlotheim; Böse and Schlosser, p. 196, pl. 18, figs. 7, 8.
- ?Reference Böse and Schlosser1900
Rhynchonella aff. alberti Oppel; Böse and Schlosser, p. 193, pl. 18, fig. 1.
- Reference Principi1910
Rhynchonella briseis Gemmellaro; Principi, p. 78, pl. 3, fig. 3.
- ?Reference Dareste de la Chavanne1920
Rhynchonella briseis Gemmellaro; Dareste de la Chavanne, p. 15, pl. 1, fig. 3; pl. 3, fig. 2.
- Reference Ager1958
Cirpa briseis (Gemmellaro); Ager, p. 52, text-fig. 28.
- Reference Sacchi Vialli and Cantaluppi1967
Cirpa fronto briseis Sacchi Vialli and Cantaluppi, p. 73, pl. 11, figs. 1–3.
- ?Reference Sacchi Vialli and Cantaluppi1967
Prionorhynchia aff. latifrons (Stur); Sacchi Vialli and Cantaluppi, p. 77, pl. 12, fig. 1.
- Reference Benigni1978
Cirpa briseis (Gemmellaro); Benigni, p. 139, pl. 14, fig. 1.
- Reference Giovannoni1981
Cirpa briseis (Gemmellaro); Giovannoni, p. 207, pl. 2, figs. 4–6.
- Reference Alméras and Elmi1987
Cirpa briseis (Gemmellaro); Alméras and Elmi, p. 50, pl. 3, fig. 1.
- Reference Alméras and Elmi1987
Cirpa briseis (Gemmellaro) morpho langi Alméras and Elmi, p. 50, pl. 3, figs. 2–5.
- ?Reference Manceñido, Pálfy and Vörös1993
Cirpa cf. briseis (Gemmellaro); Manceñido, p. 83, pl. 1, fig. 6.
- Reference Iñesta1999
Cirpa briseis (Gemmellaro); Iñesta, p. 15, pl. 1, fig. 6.
- ?Reference Alméras and Fauré2000
Cirpa briseis (Gemmellaro); Alméras and Fauré, p. 104, pl. 11, figs. 7–10.
- Reference Manceñido2002
Cirpa briseis (Gemmellaro); Manceñido et al., p. 1264, fig. 861 (1).
- Reference Vörös, Szabó, Dulai, Szente, Ebli and Lobitzer2003
Cirpa briseis (Gemmellaro); Vörös et al., p. 70, pl. 6, figs. 13–15.
- Reference Vörös, Szabó, Dulai, Szente, Ebli and Lobitzer2003
Cirpa cf. briseis (Gemmellaro); Vörös et al., p. 78, pl. 8, figs. 18, 19.
- Reference Baeza-Carratalá2004
Cirpa briseis (Gemmellaro); Baeza-Carratalá, p. 211, fig. 2(1).
- ?Reference Alméras, Elmi and Fauré2007
Cirpa briseis (Gemmellaro); Alméras et al., p. 46.
- Reference Baeza-Carratalá2008
Cirpa briseis (Gemmellaro); Baeza-Carratalá., p. 154, pl. 4, figs. 5–9.
- Reference Mandl, Dulai, Schlögl, Siblík, Szabó, Szente and Vörös2010
Cirpa briseis (Gemmellaro); Mandl et al., p. 92, pl. 2, fig. 1; pl. 7, fig. 1.
- Reference Comas-Rengifo and Goy2010
Cirpa briseis (Gemmellaro); Comas-Rengifo and Goy, p. 12, pl. 1, figs. 10, 11.
- ?Reference Vörös and Kandemir2011
Cirpa cf. briseis (Gemmellaro); Vörös and Kandemir, p. 353, fig. 4 (3).
- non Reference Rodrigo2011
Cirpa cf. briseis (Gemmellaro); Rodrigo, p. 78, pl. 1, figs. 2, 3.
- Reference Alméras and Cougnon2013
Cirpa briseis (Gemmellaro); Alméras and Cougnon, p. 55, pl. 5, fig. 10.
- Reference Alméras and Fauré2013
Cirpa briseis (Gemmellaro); Alméras and Fauré, p. 32, pl. 1, fig. 8.
- Reference Baeza-Carratalá2013
Cirpa briseis (Gemmellaro); Baeza-Carratalá., p. 84, fig. 5 (4).
- Reference Alméras, Cougnon, Guibbert and Fauré2014
Cirpa briseis (Gemmellaro); Alméras et al., p. 20, pl. 2, fig. 2.
- Reference Alméras, Cougnon, Guibbert and Fauré?2014
Cirpa briseis (Gemmellaro); Alméras et al., p. 20, pl. 2, fig. 3.
- Reference Baeza-Carratalá, García Joral and Tent-Manclús2016b
Cirpa briseis (Gemmellaro); Baeza-Carratalá et al., p. 248, fig. 4 (1).

Figure 3. Some representative Pliensbachian species of Cirpa from the External Subbetic paleomargin. Each specimen is ordered consecutively in dorsal, anterior, and lateral views. (1–14) Cirpa lucentina n. sp. (1–3) Holotype, specimen CCA.8.Clat.1 from Cerro de La Cruz; (4–6) specimen O.5.B.12.4 from JdC collection; (7–9) specimen I.11.T19(19).1 from Sierra de Algayat; (10–12) specimen CCA.8.Clat.2 from Cerro de La Cruz; (13, 14) specimen I.11.T9(9).2 from Sierra de Algayat. (15–28) Cirpa briseis (Gemmellaro, Reference Gemmellaro1874). (15–17) Specimen CCA.8.Cbri.1 from Cerro de La Cruz; (18–20) specimen I.12.24.2 from Sierra de Algayat; (21, 22) specimen O.8.23.T1.5 from Sierra de la Espada; (23–25) specimen I.12.3.2 from Sierra de Algayat; (26–28) specimen I.13.B5.5 from Sierra de Algayat. (29–37) Cirpa planifrons (Ormós, Reference Ormós1937). (29–31) Specimen CCA.8.C.pla.1 from Cerro de La Cruz; (32–34) specimen CCA.8.C.pla.2 from Cerro de La Cruz; (35–37) specimen Z1B.Clat.1 from Sierra de Orts. Scale bar = 1 cm.
Holotype
The original type material, deposited in the collections of the University of Palermo, was described by Gemmellaro (Reference Gemmellaro1874, pl. 11, figs. 19–22) from the Pliensbachian of Sicily (“Terebratula Aspasia beds”).
Occurrence
As can be deduced from the synonymy, C. briseis is pervasive and widespread from Pontides to Algeria throughout different Tethyan biochoremas. Early records of this species were cited in the “Middle Lias” from the Italian basins, the pre-Alps, Greece, and the Sinemurian–Pliensbachian of Schafberg (Austria), among many other records. The occurrence of this taxon is remarkable in the Domerian (Margaritatus Zone, Stokesi Subzone) from the Pyrenees; the late Domerian (Spinatum Zone) from the French Alps; Pliensbachian (Margaritatus Zone) from Quercy (Alméras and Fauré, Reference Alméras and Fauré2013) and Pliensbachian (Spinatum Zone) from the SE French Central Massif (Alméras and Elmi, Reference Alméras and Elmi1987); and the latest Pliensbachian from Eastern Pontides (Vörös and Kandemir, Reference Vörös and Kandemir2011). In the peri-Iberian platform system, it is recorded in the late Pliensbachian from the Subbetic area (Azéma, Reference Azéma1977; Iñesta, Reference Iñesta1988; Baeza-Carratalá, Reference Baeza-Carratalá2013); late Pliensbachian from the Prebetic/Subbetic transition (Baeza-Carratalá et al., Reference Baeza-Carratalá, García Joral and Tent-Manclús2016b), and in the Davoei–Margaritatus chronozones (Pliensbachian) from the Asturian basin (Comas-Rengifo and Goy, Reference Comas-Rengifo and Goy2010).
Description
Medium-sized dorsibiconvex shell, usually wider than long, with the maximum width and thickness shifted toward the anterior third of the shell. Dorsal outline is triangular to subpentagonal, with a rounded anterior margin. The beak is strong, suberect, and shows a small pedicle foramen and poorly developed beak ridges. The flanks of the shell show shallow and relatively narrow planareas. Lateral commissure is slightly arcuate near the beak, then running straight to the anterior margin, tilted with ventral orientation. Anterior commissure is uniplicate with a marked subtrapezoidal dorsal median fold, occasionally rendering a subcynocephalous profile to the shell. Ribbing pattern consists of 7–12 sharp and spaced ribs (3–4 of which are present on the median fold), triangular in cross-section, running along the entire shell length without bifurcation.
The internal structure of this species (Fig. 4) shows a subrectangular delthyrial cavity in cross-section, where a strong pedicle collar is visible and the double deltidial plates, distinctive of several taxa arranged into the genus Cirpa, are present (Fig. 4). Dental plates are ventrally convergent to subparallel. Hinge teeth are massive and crenulated, and are inserted in rather deep and crenulated sockets. The dorsal median septum is very short with no detectable evidence of a septalium. Hinge plates fused in the early stages (Fig. 4), ventrally arcuate to horizontal. Crural development is incipiently hamiform, but with a particularly short dorso-ventral expansion. Thus, initially, the crural bases show a markedly triangular cross-section with bracket-shaped crural progress and endings, reaching the total crural architecture around one-third of the shell-length. Secondary layer of the shell shows an eurinoid pattern, with rhombic to subrectangular cross-section of coarse calcite fibers ~70–80 μm wide and 30 μm thick (Fig. 5.3).

Figure 4. Internal structure of Cirpa briseis (Gemmellaro, Reference Gemmellaro1874) from the Pliensbachian (Eastern Prebetic); serial sections orientated with the ventral valve up. (1) Specimen CCC-3 in which serial sections were performed; (2) transverse serial sections through the same specimen (distance from the apex in mm); (3–7) photomicrographs of acetate peels from the same specimen: (3) section at 1.40 mm showing distinctive pedicle collar in the upper part; (4) section at 1.50 mm showing the short dorsal median septum; (5, 6) sections at 1.70 and 2.60 mm, respectively, showing progression of the fused hinge plates from the earlier stages; (7) section at 2.90 mm showing crural bases with an incipiently hamiform development. (8) Specimen CCB.5.2 in which serial sections were performed; (9) transverse serial sections through CCB.5.2 (distance from the apex in mm); (10–15) photomicrographs of acetate peels from CCB.5.2: (10) section at 0.80 mm showing traces of pedicle collar and dental plate; (11, 12) sections at 2.00 and 2.20 mm, respectively, showing fused hinge plates and the hinge teeth inserted in deep sockets; (13–15) partial sections at 2.60, 3.10, and 3.80 mm, respectively, corresponding with the hamiform crural development. Scale bars = 1 cm (1, 8), 2 mm (2, 9), 1 mm (3–7, 10, 13–15), and 0.5 mm (11, 12).
Figure 5. Eurinoid microstructure of the secondary layer of the shell in some wellerelloid taxa analyzed. (1) Cirpa planifrons (Ormós, Reference Ormós1937), section at 2.20 mm from the apex in the specimen CCA.8.Cpla.X. (2) Cirpa lucentina n. sp., section at 2.30 mm from the apex in the specimen I.12.T26(26).1. (3) Cirpa briseis (Gemmellaro, Reference Gemmellaro1874), section at 2.00 mm from the apex in the specimen CCB5.2. (4) Salgirella alberti (Oppel, Reference Oppel1861), section at 2.40 mm from the apex in the specimen I.12.T26(26).2. All scale bars represent 50 μm.
Material
The studied sample of this species includes 248 mostly articulated and differently preserved specimens from La Algueda (2 specimens), Tarabillo (9), Sierra de Orts (3), Cerro de la Cruz-1 (89), Cerro de la Cruz-2 (129), Miguelín quarry (6), La Mola (10), and supplemented by 32 specimens from the JdC collection derived from Sierra de Algayat, Sierra de la Espada, and Collado de La Campana.
Remarks
In addition to the main biometric ratios, intraspecific variability of C. briseis in the Betic domain mainly lies in the aforementioned presence of 7–12 ribs in the shell (3–4 on the median fold), as well as in the height of the fold, which ranges from low to subcynocephalous profiles. The rather flat ventral valve prevails in the stock of the Betic material, contributing to this cynocephalous-like profile, however several specimens exhibit higher grade of ventral convexity, as noted by previous authors (e.g., Principi, Reference Principi1910, pl. 3, fig. 3; Manceñido, Reference Manceñido, Pálfy and Vörös1993, pl. 1, fig. 6).
In contrast, Sacchi Vialli and Cantaluppi (Reference Sacchi Vialli and Cantaluppi1967) figured Cirpa fronto briseis with a lower folding pattern than the Betic individuals. As can be deduced, this acceptable range of variability in the folding pattern was noticed early, even in the original material studied by Gemmellaro (Reference Gemmellaro1874).
The typical costation pattern derived from the literature consists of 3 ribs restricted to the median fold, but an increasing number of ribs in this area is often reported. Even the type material displays specimens with 4 (Gemmellaro, Reference Gemmellaro1874, pl. 11, figs. 19, 20) and 3 ribs (Gemmellaro, Reference Gemmellaro1874, pl. 11, figs. 21, 22), which agrees with the individuals herein analyzed. Iñesta (Reference Iñesta1999) and Vörös et al. (Reference Vörös, Szabó, Dulai, Szente, Ebli and Lobitzer2003) also illustrated specimens with 4 ribs on the dorsal fold. Benigni (Reference Benigni1978) depicted one exceptional specimen matching with the concept of the species herein outlined with 15 ribs in the entire shell.
Cirpa briseis is a mainly Pliensbachian taxon conspicuously recorded in a huge number of western Tethyan localities. However, as shown in the synonymy list, this species has been misidentified occasionally as other closely related taxa belonging to the genera Cirpa and Salgirella. Some previous attributions to Rhynchonella alberti Oppel, Reference Oppel1861 (=Salgirella alberti) or related forms suitably could fit within the conspecific range of C. briseis (e.g., Böse and Schlosser, Reference Böse and Schlosser1900, pl. 18, fig. 1). Certainly, some external features in extreme morphologies of C. briseis can be compared with S. alberti, as can be deduced from the morphometrical analysis performed (see results section above), because both occupy nearby scores in the morphospace plots. However, this analysis also evinces the defining characters to separate them, such as the more-prominent smooth areas beside the dorsal fold or higher folding pattern in S. alberti, resulting in a deeper ventral sulcus. Salgirella alberti usually shows more widely expanded outlines as well.
Likewise, some specimens figured by Parona (Reference Parona1884, pl. 2, figs. 10–20; pl. 3, fig. 2) and Dareste de la Chavanne (Reference Dareste de la Chavanne1920) as Rhynchonella briseis do not fit in the species concept outlined here, and may correspond to other representatives ascribed to Cirpa (e.g., C. planifrons [Ormós, Reference Ormós1937]) or even to Prionorhynchia (e.g., Sulser and Furrer, Reference Sulser and Furrer2005, assigned Parona's referred material to Prionorhynchia calderinii [Parona, Reference Parona1880]). The same occurs with the specimen depicted by Haas (Reference Haas1884, pl. 1, fig. 6), which reveals wide and very deep Prionorhynchia-type planareas. Similarly, the variety iphimedia, erected by Di Stefano (Reference Di Stefano1891, pl. 3, figs. 14–17), does not agree with the range of variability of C. briseis due the presence of a high and narrow dorsal fold (with 5–7 ribs), exceeding the global thickness and giving a trilobate anterior outline to the shell.
The external features of Prionorhynchia aff. P. latifrons (Geyer, Reference Geyer1889) (Sacchi Vialli and Cantaluppi, Reference Sacchi Vialli and Cantaluppi1967, pl. 12, fig. 1) can be compared with C. briseis, but the serial sections of this material are not fully conclusive (Sacchi Vialli and Cantaluppi, Reference Sacchi Vialli and Cantaluppi1967, p. 77). Some doubts also exist regarding the attribution made by Alméras and Fauré (Reference Alméras and Fauré2000, especially the specimen depicted in pl. 11, fig. 10) due to the high degree of convexity of both valves, providing a globose profile to the shell. The same occurs with the Norman specimen figured by Alméras et al. (Reference Alméras, Cougnon, Guibbert and Fauré2014, pl. 2, fig. 3), which shows a lower folding pattern, imperceptible planareas, and higher convexity of the valves. Unfortunately, the western Algerian material is not figured for comparison (Alméras et al., Reference Alméras, Elmi and Fauré2007, p. 46). In the northern part of the Iberian Cordillera, Rodrigo (Reference Rodrigo2011) reported Cirpa cf. C. briseis, but some features, such as the absence of planareas, a more massive beak, the higher convexity of the valves, and costation consisting up to 19 ribs, prevent its inclusion in the genus Cirpa, pending verification concerning internal structure data. Finally, in Turkey, Vörös and Kandemir (Reference Vörös and Kandemir2011) figured a single poorly preserved specimen that could fit within the intraspecific variability herein described.
This species has been frequently incorporated among the diverse attributions to Rhynchonella variabilis (e.g., Geyer, Reference Geyer1889, pl. 4, figs. 19–21; Böse and Schlosser, Reference Böse and Schlosser1900, pl. 8, figs. 7, 8). Rhynchonella variabilis is a catch-all nominal species involving several taxa (most of them especially attributable to the genus Cirpa), inappropriately differentiated mainly by the number of ribs located on the median fold. Hence, some previous authors (e.g., Manceñido, Reference Manceñido, Pálfy and Vörös1993; Alméras and Fauré, Reference Alméras and Fauré2000; Siblík, Reference Siblík2002) endorsed Ager's (Reference Ager1959) early interpretation that R. variabilis is a nomen dubium that should be avoided unless it becomes more clearly defined in the future. Vörös et al. (Reference Vörös, Szabó, Dulai, Szente, Ebli and Lobitzer2003) also noted the broad interpretation in the literature of the species variabilis, which complicates the separation of C. briseis from other taxa included in that imprecise designation.
Some previous authors (e.g., Manceñido et al., Reference Manceñido, Owen, Dagys and Selden2002; Baeza-Carratalá, Reference Baeza-Carratalá2008) have regarded Cirpa primitiva (De Gregorio, Reference De Gregorio1930) as a junior subjective synonym of C. briseis. However, Vörös and Kandemir (Reference Vörös and Kandemir2011) seem not to fully agree with this opinion, arguing that many nominal species assigned to Cirpa only show minor differences, even including the original definition of the genus synonymized with C. briseis. In this work, it is preferred not to include C. primitiva in the synonymy list of C. briseis, thus retaining C. primitiva as a valid type species of the genus, given that the material of the present research does not allow clarifying this nominal topic.
Cirpa briseis can be easily differentiated from Cirpa langi Ager, Reference Ager1958, because this latter species shows more ribs, wider expanded outline, and much flatter valves. Cirpa langi carpathica (Siblík, Reference Siblík1966) shows coarser ribs, narrow folding pattern, and subquadrate anterior margin with straight lateral flanks. Cirpa lucentina n. sp. also has flatter valves and more ribs that are occasionally bifurcated. Cirpa slovenica Siblík, Reference Siblík1967, is oversized, with markedly elliptical anterior outline, and 4–7 ribs arranged on the median fold.
Cirpa fallax (Deslongchamps, Reference Deslongchamps1862)
Figure 6.7–6.18
- ?Reference Deslongchamps1858
Rhynchonella egretta (Nobis); Deslongchamps, p. 164, pl. 4, figs. 4–6.
- Reference Deslongchamps1862
Rhynchonella fallax Deslongchamps, p. 267, pl. 3, figs. 1–5.
- non Reference Davidson1884
Rhynchonella fallax Deslongchamps; Davidson, p. 275, pl. 20, figs. 4, 5.
- non Reference Buckman1918
Rudirhynchia fallax (Deslongchamps); Buckman, p. 45.
- non Reference Muir-Wood1928
Rudirhynchia? fallax (Deslongchamps); Muir-Wood, p. 249, fig. 6.
- non Reference Ager1958
Rudirhynchia fallax (Deslongchamps); Ager, p. 83.
- non Reference Ager1962
Rudirhynchia fallax (Deslongchamps); Ager, pl. 8, fig. 1.
- Reference Alméras, Copper and Jin1996
Pseudogibbirhynchia fallax (Deslongchamps); Alméras, p. 8, pl. 1, figs. 4–6.
- Reference Comas-Rengifo, Duarte, García Joral and Goy2013
Cirpa fallax (Deslongchamps); Comas-Rengifo et al., fig. 2.
- Reference Comas-Rengifo, Duarte, Félix, García Joral, Goy and Rocha2015
Cirpa fallax (Deslongchamps); Comas-Rengifo et al., fig. 3: 7.

Figure 6. Some representative Early Jurassic species of Cirpa from the Lusitanian, Internal Subbetic, and easternmost Subbetic paleomargins. Each specimen is ordered consecutively in dorsal, anterior, and lateral views (1–18) or in dorsal, lateral, and anterior views (19–21). (1–6) Cirpa cf. C. slovenica Siblík, Reference Siblík1967; (1–3) specimen O.5.B.10.1; (4–6) specimen O.5.B.10.2 from the JdC collection. (7–18) Cirpa fallax (Deslongchamps, Reference Deslongchamps1862) from the Toarcian of Portugal; (7–9) specimen PT.33.1 from the Polymorphum Zone of Peniche; (10–18) specimens FC.6.12, FC.12.7, and FC.12.2, respectively, from the Polymorphum Zone of Fonte Coberta. (19–21) Cirpa subcostellata (Gemmellaro, Reference Gemmellaro1882), specimen SG1.CS1 from the Sinemurian of Sierra Gorda (Internal Subbetic, Granada). Scale bar = 1 cm.
Holotype
The holotype and possible syntypes originally studied and drawn by Deslongchamps (Reference Deslongchamps1858) come from the locality of May (South-Caen, Normandy, France). According to Deslongchamps (Reference Deslongchamps1858, p. 139–154), the original Rh. fallax was found in deposits filling an Ordovician relief just below the “Couche à Leptaena”. These specimens are missing because they were housed in the collections of the Museum of Caen, which was destroyed in World War II (Alméras et al., Reference Alméras, Cougnon, Guibbert and Fauré2014). This work illustrates several hypotypes (Fig. 6.7–6.18), including specimen FC.12.7 (Fig. 6.13–6.15), which is the individual showing the most relevant diagnostic criteria. A second sectioned hypotype (FC.12.31) is shown in Figure 7. The “Couche à Leptaena” are equivalent in age to the “Marly Limestones with Leptaena Fauna” (MLLF) Member of the São Gião Formation from the Polymorphum Zone at the Fonte Coberta outcrop (Portugal) (Duarte et al., Reference Duarte, Comas-Rengifo, García Joral, Goy, Míguez-Salas and Rodríguez-Tovar2018), from which the proposed hypotypes come. Both specimens are stored within the DPUCM collections.

Figure 7. Internal structure of Cirpa fallax (Deslongchamps, Reference Deslongchamps1862) from the Toarcian (Lusitanian Basin); serial sections orientated with the ventral valve up. (1) Specimen FC.12.31 in which serial sections were performed; (2) transverse serial sections through the same specimen (distance from the apex in mm); (3–5) photomicrographs of acetate peels from the same specimen: (3) section at 2.50 mm showing the fused hinge plates; also notice the interlocked dentition and the starting of the crural bases; (4) section at 1.10 mm showing the doubled (“buttressed”) deltidial plates; (5) section at 3.80 mm with a detail of the eurinoid microstructure of the shell. Scale bars = 1 cm (1), 2 mm (2), 1 mm (3, 4) and 0.5 mm (5).
Occurrence
Rhynchonella fallax Deslongchamps, Reference Deslongchamps1862, was originally described from the Middle Lias from Calvados (NW France). However, Alméras (Reference Alméras, Copper and Jin1996, p. 8) stated that, in fact, this material comes from the lower Toarcian. Its record in the peri-Iberian platforms is limited to the Polymorphum Zone of the Lusitanian Basin (corresponding to the Tenuicostatum Zone in the northwestern European areas).
Description
Small- to medium-sized equibiconvex shell, with both valves rather flat, and sub-triangular outline in dorsal view. Shell wider than long, with the maximum width in the anterior third of the shell. The subrectangular anterior profile with flattened and truncated anterior margin is representative of this species of Cirpa. Beak suberect, with a relatively large foramen, often rimmed. Lateral commissure is straight and the anterior one is uniplicate, showing a wide and low dorsal median fold, sub-rectangular in outline. Ribbing pattern consists of 15–24 (x̄ = 18.7) sharp and triangular ribs (4–8 on the median fold), frequently bifurcated in their posterior part.
Cirpa fallax shows a subrectangular to trapezoidal delthyrial cavity in cross-section, with short dental plates and double deltidial plates (Fig. 7.2, 7.4). Hinge teeth are massive and crenulated, supplemented by small denticula; teeth are inserted in crenulated sockets as well. The dorsal median septum is barely visible and septalium is absent. Hinge plates fused, parallel, and horizontal (Fig. 7.2, 7.3). Crural development is hamiform, crural bases with triangular cross-section, progressing anteriorly with short ventral development. Secondary layer of the shell shows an eurinoid pattern with rhombic cross-sectional outline of the calcite fibers (Fig. 7.5).
Material
Forty-five whole specimens (31 mensurable) from Fonte Coberta (Rabaçal) (41), Peniche (2), and Ribeiro (Coimbra) (2) outcrops, all of them belonging to the Lusitanian Basin (Portugal).
Remarks
Cirpa fallax is distinguished from other species of Cirpa by its large number of strong, often bifurcated ribs, and its lower lateral profile. Alméras (Reference Alméras, Copper and Jin1996, p. 8) assigned three specimens from Peniche with these external features to Pseudogibbirhynchia fallax, due to their close similarity to specimens from Rabaçal attributed to Pseudogibbirhynchia moorei (Davidson in Ager, Reference Ager1962); however, the paucity of specimens prevented the study of internal structures. Previously, Buckman (Reference Buckman1918, p. 45) included the English forms attributed by Davidson (Reference Davidson1884) to R. fallax in his new genus Rudirhynchia. This determination was subsequently followed by Muir-Wood (Reference Muir-Wood1928) and Ager (Reference Ager1958, Reference Ager1959, Reference Ager1962), but lacked data on the internal structure. However, Ager (Reference Ager, Adams and Ager1967, p. 163), after examination of new material from Somerset, considered these English forms to be upper Sinemurian in age, different from the Norman species, and even attributed this material to Prionorhynchia greppini (Oppel, Reference Oppel1861).
Three sectioned specimens from Rabaçal and Peniche reveal the characteristic internal features of the genus Cirpa, particularly the fused cardinal plates and double strengthened deltidial plates (Fig. 7). This led Comas-Rengifo et al. (Reference Comas-Rengifo, Duarte, García Joral and Goy2013, Reference Comas-Rengifo, Duarte, Félix, García Joral, Goy and Rocha2015) to reconsider the generic position of Rh. fallax, arranging the Portuguese material in the genus Cirpa.
Specimens of the original “Rhynchonella” fallax, which are only known from drawings, are probably missing, as unfortunately occurred with many parts of the Deslongchamps collections, because of the destruction of the Caen Museum in World War II (Alméras et al., Reference Alméras, Cougnon, Guibbert and Fauré2014). Attribution of the specimens studied to “Rh.” fallax is based on the conclusions of Alméras (Reference Alméras, Copper and Jin1996). Nevertheless, the nominal species “Rhynchonella egretta” by Deslongchamps (Reference Deslongchamps1858, p. 164, pl. 4, figs. 4–6) from the same “Leptaena Beds” of May (Calvados) could also be related to the Portuguese specimens. Drawings of the specimens of this taxon show the bifurcated (or intercalated) ribs that are common in C. fallax from Portugal and equally low lateral profile, although the median fold is much more pronounced. In the original description, this material is associated with numerous species of thecideides, koninckinides, spiriferinides, terebratulides, and rhynchonellides, including Pseudokingena deslongchampsi (Davidson, Reference Davidson1850), Koninckella liasina (Bouchard in Davidson and Morris, Reference Davidson and Morris1847), and Nannirhynchia pygmaea (Morris in Davidson and Morris, Reference Davidson and Morris1847), which is similar to the assemblage where C. fallax is recorded in the Lusitanian Basin. Because the original type specimens are missing due to the destruction of the Museum of Caen, it remains unclear whether the Portuguese Cirpa and Rh. egretta are conspecific, thus we retain the specific attribution proposed by Alméras (Reference Alméras, Copper and Jin1996).
Cirpa lucentina new species
Figure 3.1–3.14, 8
- Reference Jiménez de Cisneros1923
Rhynchonella aff. variabilis Schlotheim; Jiménez de Cisneros (part) p. 26, pl. 5, fig. 14.
- Reference Jiménez de Cisneros1923
Rhynchonella latifrons Stur; Jiménez de Cisneros, p. 36.
- ?Reference Sacchi Vialli and Cantaluppi1967
Prionorhynchia latifrons (Stur); Sacchi Vialli and Cantaluppi, p. 76, pl. 11, figs. 7, 8 (part; not Prionorhynchia aff. latifrons, pl. 12, fig. 1).
- Reference Baeza-Carratalá2004
Cirpa latifrons (Stur in Geyer); Baeza-Carratalá, p. 211, fig. 2(2).
- Reference Baeza-Carratalá2008
Cirpa aff. latifrons (Stur in Geyer); Baeza-Carratalá, p. 164, pl. 4, figs. 1–4.
- Reference Baeza-Carratalá2013
Cirpa aff. latifrons; Baeza-Carratalá, p. 80, fig. 3A.
- Reference Baeza-Carratalá, García Joral and Tent-Manclús2016b
Cirpa aff. latifrons; Baeza-Carratalá et al., p. 248, fig. 4(7).
Holotype
Specimen CCA.8.Clat.1 (Fig. 3.1–3.3). Dimensions (in mm): L: 16.73; W: 18.31; T: 9.04. Type locality: Cerro de la Cruz de La Romana, Alicante Province, Betic Cordillera, Spain. Red crinoidal grainstone member of the Gavilán Formation; Pliensbachian. Deposited in the collections of the DCTMA (University of Alicante, Spain).
Diagnosis
Medium-sized multicostate Cirpa with rather flat valves. Beak small with short and slightly depressed planareas. Anterior commissure uniplicate in a wide dorsal median fold; with numerous ribs, occasionally bifurcate; pedicle collar present, double triangular deltidial plates; fused subhorizontal hinge plates, hamiform crura.
Occurrence
In the Subbetic Domain, the material updated as C. lucentina n. sp. was cited by Jiménez de Cisneros (Reference Jiménez de Cisneros1923) in the “Middle Lias.” Baeza-Carratalá (Reference Baeza-Carratalá2013) restricted the distribution range of this taxon in the Subbetic and the Prebetic/Subbetic transitional zones (Baeza-Carratalá et al., Reference Baeza-Carratalá, García Joral and Tent-Manclús2016b) to the late Pliensbachian.
Description
Medium-sized biconvex shells with both valves rather flat. Shell wider than long and the thickness is about half its width. The maximum width lies around the mid-length and the maximum thickness is shifted toward the posterior third of the shell. Dorsal outline is triangular with a rounded anterior margin. The beak is small and strong, suberect, and shows a minute pedicle foramen. Narrow, faintly defined beak ridges bordering slightly depressed planareas that are poorly developed on the flanks. Commissure is straight laterally and uniplicate at anterior margin, showing a low, arcuate, and wide dorsal median fold. Multicostate shell, displaying 13–16 ribs running along the entire shell length, triangular in cross-section (4–6 of which are present on the median fold). It is not unusual to detect bifurcations originating from the posterior third of the shell.
In its internal structure (Fig. 8), posterior subelliptical delthyrial cavity and semicircular umbonal cavities are visible. Pedicle collar present. Double deltidial plates, triangular in cross-section. Dental plates are straight and subparallel. Hinge teeth and sockets are crenulated, also developing small denticula. Dorsal median septum very short. Hinge plates are slender and fused, parallel to the commissural plane. They initially show a slight ventral orientation, anteriorly becoming straight and subparallel. Hamiform crural development, with relatively large comma-shaped crural endings (Fig. 8). Secondary layer of the shell shows an eurinoid pattern, featuring fibers with rhombic/subquadrate outline in cross section, ~60–80 μm wide and 30–50 μm thick (Fig. 5.2).

Figure 8. Internal structure of Cirpa lucentina n. sp. from the Pliensbachian (Eastern Prebetic); serial sections orientated with the ventral valve up. (1) Specimen I.12.T26(26).1 in which serial sections were performed; (2) transverse serial sections through the same specimen (distance from the apex in mm); (3–5) photomicrographs of acetate peels from the same specimen: (3) section at 0.30 mm showing distinctive pedicle collar between dental plates; (4) section at 1.40 mm showing the short dorsal median septum; (5) section at 1.70 mm showing fused hinge plates and insertion of hinge teeth in sockets. Scale bars = 1 cm (1), 2 mm (2), and 1 mm (3–5).
Etymology
From the Latin, Lucentum, the ancient toponym of Alicante, the region where this species is recorded for the first time.
Material
Fifty-six specimens sampled from Cerro de la Cruz-1 (23), Cerro de la Cruz-2 (32), and La Mola (1); supplemented by 58 shells from the JdC collection derived from Rincón de Egea, Sierra de la Espada, Sierra de Algayat, Cerro de la Cruz, Moleta de Togores, and Collado de la Campana outcrops.
Remarks
Intraspecific variability of Cirpa lucentina n. sp. mainly lies in the number of costae, which is increased by bifurcation in some individuals. Moreover, while the valves are consistently flat, several specimens show slightly dorsibiconvex profiles (e.g., Jiménez de Cisneros, Reference Jiménez de Cisneros1923). In addition, the anterior folding shape can vary from the representative arcuate/semicircular to straight/subrectangular outlines.
As can be deduced from the synonymic list, specimens of Cirpa lucentina n. sp. were related and assimilated as possible Betic counterparts of the species Rhynchonella latifrons Geyer, Reference Geyer1889, and were assigned in open nomenclature to Cirpa aff. C. latifrons in the latest papers (Baeza-Carratalá, Reference Baeza-Carratalá2004, Reference Baeza-Carratalá2008, Reference Baeza-Carratalá2013; Baeza-Carratalá et al., Reference Baeza-Carratalá, García Joral and Tent-Manclús2016b). This attribution was based upon the external features (i.e., folding pattern, flattened valves, even specimens bearing bifurcated ribs), except for the development of (often short) planareas in the Betic specimens, which apparently are comparable with the records of the Austrian Alps where the types of Rhynchonella latifrons were defined by Geyer (Reference Geyer1889). In this sense, Rh. latifrons was tentatively attributed to Cirpa by several previous authors (Dulai, Reference Dulai1992, Reference Dulai2003; Böhm et al., Reference Böhm, Ebli, Krystin, Lobitzer, Rakús and Siblík1999, Vörös et al., Reference Vörös, Szabó, Dulai, Szente, Ebli and Lobitzer2003; Baeza-Carratalá, Reference Baeza-Carratalá2004). This attribution to the genus Cirpa was consistent with the internal structure revealed by the Subbetic stock (Fig. 8), with double deltidial plates, fused hinge plates, and hamiform crura. Thus, Baeza-Carratalá (Reference Baeza-Carratalá2008, Reference Baeza-Carratalá2013) proposed the combination Cirpa aff. C. latifrons.
Unravelling the taxonomy of lowermost Jurassic multicostate rhynchonellids, Tomašových (Reference Tomašových2006) thoroughly revised the attributions of Rh. latifons (Geyer, Reference Geyer1889). The very comprehensive analysis of the internal structure in such specimens revealed that the Hettangian–Sinemurian “true latifrons” from the Western Carpathians and the Austrian Alps evolved disjoint hinge plates inclined forming a sessile septalium, no pedicle collar, and subfalciform crura. This evidence led to erection of a new genus (Jakubirhynchia Tomašových, Reference Tomašových2006), with Jakubirhynchia latifrons (Geyer, Reference Geyer1889) as type species, separate from Cirpa, even at the Superfamily level (Cirpa = Wellerelloidea; Jakubirhynchia = Pugnacoidea).
Bearing in mind that the Betic material undoubtedly can be placed in the genus Cirpa, it should be split from the J. latifrons stock, and we consider it herein as new species. Additionally, both biostratigraphical ranges are unconnected since Jakubirhynchia latifrons is regarded hitherto as a representative species from the Hettangian–Sinemurian, while C. lucentina n. sp. is recorded in the late Pliensbachian.
On the other hand, the material recorded in the easternmost Subbetic and the Prebetic/Subbetic transitional areas of La Mola region differs from several comparable species assigned to Cirpinae, such as Calcirhynchia plicatissima (Quenstedt, Reference Quenstedt1852), which shows higher convexity on both valves and narrower dorsal outline. Cirpa planifrons shows anteriorly truncated dorsal outline and narrower fold, and Cirpa subcostellata (Gemmellaro, Reference Gemmellaro1882) has fewer ribs overall. Finally, among the Jakubirhynchia representatives, Jakubirhynchia? fascicostata (Uhlig, Reference Uhlig1879) externally differs in having more convex valves and numerous ribs.
Possible equivalents of the new species herein erected may be the specimens assigned to Prionorhynchia latifrons by Sacchi Vialli and Cantaluppi (Reference Sacchi Vialli and Cantaluppi1967), which show a narrower dorsal median fold, fitting within the intraspecific variability of this taxon. Moreover, the partial serial sections performed by the aforementioned authors (Sacchi Vialli and Cantaluppi, Reference Sacchi Vialli and Cantaluppi1967, p. 76, text-fig. 4) might be attributable to the genus Cirpa, but they are not conclusive because the entire crural development is not clear.
Cirpa planifrons (Ormós, Reference Ormós1937)
Figure 3.29–3.37
- Reference Ormós1937
Rhynchonella planifrons Ormós, p. 41, pl. 1, fig. 19.
- Reference Siblík1993a
Cirpa planifrons (Ormós); Siblík, p. 967, pl. 1, figs. 1–3.
- Reference Siblík, Pálfy and Vörös1993b
Cirpa planifrons (Ormós); Siblík, p. 130, pl. 2, fig. 6.
- Reference Böhm, Ebli, Krystin, Lobitzer, Rakús and Siblík1999
Cirpa planifrons (Ormós); Böhm et al., p. 196, pl. 29, figs. 7, 9.
- Reference Vörös, Szabó, Dulai, Szente, Ebli and Lobitzer2003
Cirpa planifrons (Ormós); Vörös et al., p. 70, pl. 6, figs. 19, 20.
- Reference Dulai2003
Cirpa aff. planifrons (Ormós); Dulai, p. 20, pl. 2, figs. 11–16.
- ?Reference Alméras, Elmi and Fauré2007
Cirpa? planifrons (Ormós); Alméras et al., p. 44.
- Reference Baeza-Carratalá2008
Cirpa planifrons (Ormós); Baeza-Carratalá, p. 171, pl. 7, fig. 6.
- Reference Siblík and Lobitzer2008
Cirpa planifrons (Ormós); Siblík and Lobitzer, p. 65, pl. 1, fig. 9.
- ?Reference Mandl, Dulai, Schlögl, Siblík, Szabó, Szente and Vörös2010
Cirpa planifrons (Ormós); Mandl et al., p. 91, pl. 2, fig. 2.
Holotype
The holotype of this species was described and figured by Ormós (Reference Ormós1937, p. 41, pl. 1, fig. 19). Sinemurian (Oxynotum Zone) from the “Unteren Lias-Schichten,” Kékhegy, Bakony Mts., Hungary.
Occurrence
The original Rhynchonella planifrons Ormós (Reference Ormós1937) was cited in the Sinemurian (Oxynotum Chronozone) from Hungary. Most of the occurrences of this species were reported from the Sinemurian: the Marmorea Zone from Northern Calcareous Alps (Siblík, Reference Siblík1993a); from Schafberg (Austria) (Vörös et al., Reference Vörös, Szabó, Dulai, Szente, Ebli and Lobitzer2003); and from the Hettangian–late Sinemurian of the Bakony Mts. (Hungary) (Dulai, Reference Dulai2003; Vörös and Dulai, Reference Vörös and Dulai2007). Mandl et al. (Reference Mandl, Dulai, Schlögl, Siblík, Szabó, Szente and Vörös2010) recorded this taxon in the Sinemurian–Pliensbachian? from Austria. The occurrence in the peri-Iberian platforms is limited to the Pliensbachian from the eastern Subbetic domain.
Description
Medium-sized equibiconvex shell, with both valves rather flat and with triangular outline in dorsal view. Shell wider than long, except for several specimens that show nearly equidimensional W/L ratios. Maximum width lies in the anterior third of the shell, slightly shifted toward the anterior margin. The subrectangular outline in the anterior view, with flattened and truncated anterior margin, is representative of this species of Cirpa. The beak is small, strong, suberect, and shows a minute pedicle foramen and poorly developed beak ridges. Well-developed but shallow planareas. Lateral commissure is straight; anterior commissure is uniplicate, showing a wide dorsal median fold, rectangular in outline. Ribbing pattern consists of 10–14 sharp, narrow, and triangular ribs (4–6 on the median fold).
Cirpa planifrons shows a subrectangular delthyrial cavity in cross-section, with a strong pedicle collar and well-developed deltidial plates (Fig. 9). Dental plates are short and subparallel. Hinge teeth are massive and crenulated, supplemented by small denticula, teeth are inserted in concomitantly crenulated sockets. The dorsal median septum is ephemeral; it is observed along 10 μm in cross-section, and the septalium is absent (Fig. 9.2). Hinge plates persistently fused, parallel, and horizontal (Fig. 9.2, 9.7). Crural development is hamiform, crural bases with triangular cross-section, progressing anteriorly with ventral development and revealing endings with inverted U-shaped section. Secondary layer of the shell shows an eurinoid pattern, with rhombic cross-sectional outline of the calcite fibers ~50–70 μm wide and 20–25 μm thick (Fig. 5.1).
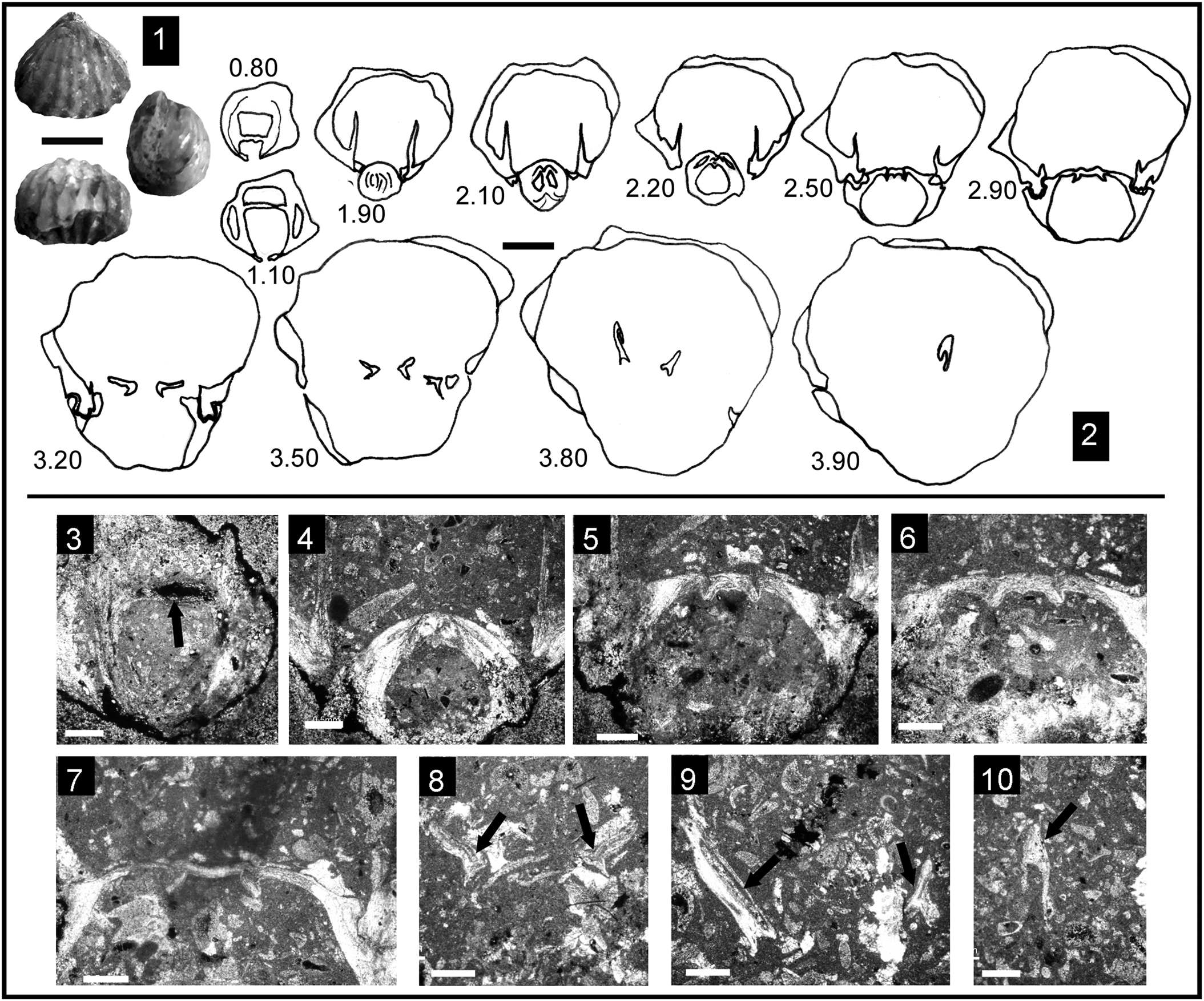
Figure 9. Internal structure of Cirpa planifrons (Ormós, Reference Ormós1937) from the Pliensbachian (Eastern Prebetic); serial sections orientated with the ventral valve up. (1) Specimen CCA.8.Cpla.X in which serial sections were performed; (2) transverse serial sections through the same specimen (distance from the apex in mm); (3–10) photomicrographs of acetate peels from the same specimen: (3) section at 1.00 mm showing pedicle collar (black arrow) and well-developed deltidial plates; (4–7) sections at 2.30, 2.50, 2.70, and 2.90 mm, respectively, showing evolution of the fused hinge plates and progression of the articulation with the features of hinge teeth and sockets; (8–10) sections at 3.50, 3.80, and 4.10 mm, respectively, showing hamiform crural (black arrows) development progressing ventrally anteriorly with inverted U-shaped sections distally. Scale bars = 1 cm (1), 2 mm (2), and 0.5 mm (3–10).
Material
Eleven mostly poorly preserved specimens sampled from Cerro de la Cruz-1 (5), Cerro de la Cruz-2 (3), and Sierra de Orts (3), supplemented by nine specimens from the JdC collection derived from Sierra de la Espada and Sierra de Algayat.
Remarks
Cirpa planifrons shows a relatively low degree of intraspecific variability in the Subbetic material. Similar patterns of ribbing are noted both in the number of total ribs and in those confined on the median fold. Only a single specimen with one bifurcated rib was observed, as was earlier depicted in the previous literature (Siblík, Reference Siblík1993a; Vörös et al., Reference Vörös, Szabó, Dulai, Szente, Ebli and Lobitzer2003). The standard flattened valves can acquire a slight degree of convexity in some specimens, as was reported by Siblík (Reference Siblík1993a, Reference Siblík, Pálfy and Vörösb). Juvenile specimens tend to be equidimensional, deviating from the usual wider than long biometric proportion of adults. This condition is also noticed in the material recorded by Dulai (Reference Dulai2003). Mandl et al. (Reference Mandl, Dulai, Schlögl, Siblík, Szabó, Szente and Vörös2010) depicted specimens with high degree of convexity and rather developed dorsal median fold, both features influencing the folding pattern, which differs from typical specimens assigned to this species. Specimens from western Algeria (Alméras et al., Reference Alméras, Elmi and Fauré2007) cannot be compared due the absence of illustrations of the two incomplete specimens cited. Siblík (Reference Siblík1993a) and Siblík and Lobitzer (Reference Siblík and Lobitzer2008) partially sectioned this species, matching well the total progression of the internal architecture herein revealed (Fig. 9).
Cirpa planifrons differs from C. lucentina n. sp. in having a more rectangular and truncated anterior margin, as well as straight flanks. Cirpa fronto (Quenstedt, Reference Quenstedt1871) shows fewer and coarser ribs over the entire shell surface, as reported by Siblík (Reference Siblík, Pálfy and Vörös1993b) and Vörös et al. (Reference Vörös, Szabó, Dulai, Szente, Ebli and Lobitzer2003).
Cirpa cf. C. slovenica Siblík, Reference Siblík1967
Figure 6.1–6.6
- cf. Reference Siblík1966
Cirpa langi ssp. n., Siblík, p. 154 (fide Siblík, Reference Siblík1967, p. 155).
- cf. Reference Siblík1967
Cirpa slovenica Siblík, p. 155, pl. 9, figs. 1–3.
- Reference Baeza-Carratalá2008
Cirpa cf. slovenica (Siblík); Baeza-Carratalá., p. 162, pl. 5, figs. 1, 2.
Holotype
The specimen MS-164 deposited in the collection of the Geological Survey of Prague was originally designated as the holotype of Cirpa slovenica (Siblík, Reference Siblík1967, pl. 9, fig. 1) and was collected in the uppermost beds of the Domerian from the Kostelec locality.
Occurrence
Siblík (Reference Siblík1967) recorded this species in the latest Pliensbachian from Slovakia. The Subbetic material is attributed to late Pliensbachian as well.
Description
Medium- to large-sized dorsibiconvex shell, considerably wider than long. The dorsal outline is subpentagonal with a rounded anterior margin. The beak is suberect, showing a small pedicle foramen; the beak ridges are sharp in the posterior third of the shell. Well-developed smooth, narrow, and shallow planareas. Squama-glotta present near the beak (Fig. 6.6), then the lateral commissure running straight with ventral-tilted orientation. Anterior commissure is uniplicate, with a wide subtrapezoidal dorsal median fold. Fully costate shell consisting of 13–16 sharp and triangular ribs (4–7 of which occur on the median fold), without bifurcation. Growth lines are poorly visible, only on the planareas. Available material is not suitable for making serial sections.
Material
Eleven mostly fragmented specimens sampled from the Cerro de la Cruz-1 (3) and Cerro de la Cruz-2 (8); supplemented by 13 complete specimens from the JdC collection derived from Moleta de Togores and Sierra de la Espada outcrops.
Remarks
Specimens herein analyzed have been attributed to Cirpa cf. C. slovenica due to the considerable external similarity with the type material described and figured by Siblík (Reference Siblík1967), because the Subbetic material differs only in having a higher folding pattern. The Subbetic material assigned to C. cf. C. slovenica corresponds with a large morphotype of Cirpa, comparable with the largest figured specimens of C. briseis (e.g., Di Stefano, Reference Di Stefano1891). Nevertheless, the widely expanded profile and greater number of ribs (both on the shell over and on the median dorsal fold) allow separating our specimens from C. briseis.
Cirpa? subcostellata (Gemmellaro, Reference Gemmellaro1882)
Figure 6.19–6.21
- Reference Gemmellaro1882
Rhynchonella subcostellata n. sp. Gemmellaro, p. 422, pl. 31, figs. 75–78.
- ?Reference Böse1897
Rhynchonella subcostellata Gemmellaro; Böse, p. 193, pl. 14, fig. 9.
- ?Reference Haas1912
Rhynchonella subcostellata Gemmellaro; Haas, p. 246, pl. 19, fig. 20.
- Reference Dulai2003
Cirpa subcostellata (Gemmellaro); Dulai, p. 21, pl. 3, figs. 1–3.
- Reference Elmi, Alméras, Benhamou, Mekahli and Marok2003
Cirpa subcostellata (Gemmellaro); Elmi et al., p. 701, pl. 4, fig. 5.
- Reference Vörös, Szabó, Dulai, Szente, Ebli and Lobitzer2003
Cirpa subcostellata (Gemmellaro); Vörös et al., p. 71, pl. 6, figs. 13–15.
- Reference Vörös and Dulai2007
Cirpa subcostellata (Gemmellaro); Vörös and Dulai, p. 54, pl. 1, fig. 12.
- ?Reference Alméras, Elmi and Fauré2007
Cirpa subcostellata (Gemmellaro); Alméras et al., p. 45, pl. 2, figs. 8–10.
- Reference Siblík and Lobitzer2008
Cirpa? aff. subcostellata; Siblík and Lobitzer, p. 66, pl. 2, fig. 6.
- Reference Vörös2009
Cirpa? subcostellata (Gemmellaro); Vörös, p. 76, pl. 8, fig. 9.
- Reference Mandl, Dulai, Schlögl, Siblík, Szabó, Szente and Vörös2010
Cirpa? subcostellata (Gemmellaro); Mandl et al., p. 89, pl. 2, fig. 3.
- Reference Baeza-Carratalá, Dulai and Sandoval2018b
Cirpa subcostellata (Gemmellaro); Baeza-Carratalá et al., p. 373, pl. 4, fig. 5.
Holotype
The type specimen was figured in four views (dorsal, anterior, lateral, and ventral) by Gemmellaro (Reference Gemmellaro1882, pl. 31, figs. 75–78). It was collected from the “Lower Liassic” of Sicily, and is deposited in the collections of the University of Palermo.
Occurrence
Sinemurian–Early Pliensbachian from the Transdanubian Ranges (Vörös and Dulai, Reference Vörös and Dulai2007) and Sicily (Gemmellaro, Reference Gemmellaro1882); Sinemurian from Northern Calcareous Alps (Vörös et al., Reference Vörös, Szabó, Dulai, Szente, Ebli and Lobitzer2003; Mandl et al., Reference Mandl, Dulai, Schlögl, Siblík, Szabó, Szente and Vörös2010); late Sinemurian–early Pliensbachian (Raricostatum–Aenigmaticum zones) from western Algeria (Elmi et al., Reference Elmi, Alméras, Benhamou, Mekahli and Marok2003; Alméras et al., Reference Alméras, Elmi and Fauré2007). Sinemurian (Turnieri–Raricostatum? zones) from the Subbetic area (Baeza-Carratalá et al., Reference Baeza-Carratalá, Dulai and Sandoval2018b; this work).
Description
The specimens recorded present a subpentagonal, widely expanded dorsal outline, showing an uniplicate folding pattern with a low, narrow, and trapezoidal dorsal fold. The multicostate shell displays 14 densely packed and acute ribs (4 on the median fold), often bifurcate. The flanks of the shell show shallow and well-developed planareas. There is not enough material available for studying the internal structure.
Material
In the peri-Iberian platform system, this species of Cirpa is only recorded from the offshore areas of the Internal Subbetic Zone. The material also corresponds to very scarce (4) specimens derived from Sierra Gorda (Granada Province) assigned to the Sinemurian by Baeza-Carratalá et al. (Reference Baeza-Carratalá, Dulai and Sandoval2018b).
Remarks
Vörös (Reference Vörös2009) thoroughly described and discussed this taxon and we fully agree with the interpretation that this author gives to C.? subcostellata. The few differences found in the Betic material with respect to those synonymized above are the presence of lower and lesser-constrained anterior uniplication and longer interareas. We also agree with the opinion of Vörös (Reference Vörös2009) about giving an open generic nomenclature, awaiting better-preserved material to understand the internal structure of this species. On the basis of the external criteria, such as the beak features and the ribbing and folding patterns, it seems appropriate to assign this taxon provisionally to the genus Cirpa.
Genus Salgirella Moisseiev, Reference Moisseiev1936
Type species
Rhynchonella albertii Oppel, Reference Oppel1861.
Remarks
Since erection of the genus Salgirella in the “Middle Lias” of Crimea by Moisseiev (Reference Moisseiev1936), the generic usage of Salgirella, separate from Cirpa, has been widely extended principally for reporting the type species Salgirella alberti (Oppel), largely widespread in the Mediterranean and Pontic biochoremas of the western Tethys. Several other reports of Salgirella, mainly in the Mediterranean bioprovince (e.g., S.? magnicostata [Ormós, Reference Ormós1937], S.? goicoecheai Baeza-Carratalá, Reference Baeza-Carratalá2011) would suggest that the separation of both genera may be appropriate. However, Cirpa and Salgirella have been considered as possible synonyms by some previous authors (e.g., Manceñido et al., Reference Manceñido, Owen, Dagys and Selden2002). According to the original diagnosis of Moisseiev and subsequent works of this author, Salgirella is typified by pentagonal-rounded dorsal outlines, with robust and incurved beak, acute and coarse ribs, short dental plates detached early from the ventral valve, deltidial plates with undulate curvature, short and poorly developed dorsal median septum, integral fused hinge plates, and septalium present. All of these characters, together with several morphometrical parameters herein analyzed (e.g., length of smooth areas separating median fold and flanks, ribbing pattern, widely expanded profile), are distinctive enough to justify a consistent basis for its taxonomic distinction from the genus Cirpa along with some additional criteria.
Internal structure.—The internal structure of both genera is similar regarding the crural architecture, with hamiform development and initially fused hinge plates. The dorsal septum is extremely short in both genera (a bit larger in Salgirella), but Salgirella is distinguished from Cirpa by the presence of a short U-shaped septalium. Distinctive double deltidial plates of Cirpa are not so clearly evident in Salgirella (this genus exhibiting a triangular cross section of deltidial plates), but more data are required to substantiate this last feature.
External structure.—Morphometric analysis of the dataset including all peri-Iberian cirpines has revealed a stock of Salgirella with more pronounced and wider smooth areas developed alongside the dorsal median fold than in representatives of genus Cirpa. This also implies more-spaced ribs in these areas resulting a deeper corresponding ventral sulcus. These external attributes may be considered as additional diagnostic criteria for Salgirella, combined with those proposed by previous authors, such as widely expanded outlines or the beak features, among others.
Salgirella alberti (Oppel, Reference Oppel1861)
Figure 10
- Reference Oppel1861
Rhynchonella Albertii Oppel, p. 546, pl. 13, fig. 4.
- Reference Uhlig1879
Rhynchonella Albertii Oppel; Uhlig, p. 32, pl. 4, fig. 1.
- non Reference Uhlig1879
Rhynchonella Albertii Oppel var. sospirolensis; Uhlig, p. 32, pl. 4, fig. 2.
- Reference Geyer1889
Rhynchonella Alberti Oppel; Geyer, p. 43, pl. 5, figs. 14–17.
- Reference Geyer1889
Rhynchonella Alberti Oppel var. lobata; Geyer, p. 45, pl. 5, fig. 18.
- ?Reference Fucini1895
Rhynchonella Alberti Oppel; Fucini, p. 172, pl. 7, fig. 1.
- ?Reference Böse and Schlosser1900
Rhynchonella sp. aff. Alberti Oppel; Böse and Schlosser, p. 193, pl. 18, fig. 1.
- ?Reference Principi1910
Rhynchonella Alberti Oppel; Principi, p. 79, pl. 3, fig. 8.
- non Reference Haas1912
Rhynchonella n. sp. ind. ex aff. Alberti Oppel; Haas, p. 241, pl. 19(1), fig. 16.
- Reference Dareste de la Chavanne1920
Rhynchonella Albertii Oppel; Dareste de la Chavanne, p. 18, pl. 1, fig. 5.
- Reference Ormós1937
Rhynchonella alberti var. lobata Geyer; Ormós, p. 25, pl. 1, fig. 7.
- non Reference Ormós1937
Rhynchonella alberti var. minor; Ormós, p. 25, pl. 1, figs. 8, 9.
- Reference Berg, Krimholz, Moisseiev, Mjatliuk, Petrova, Pcelincev, Riabinin, Tchernyshev, Sharapova and Yakowlew1947
Salgirella alberti (Oppel); Moisseiev in Berg et al., p. 91, pl. 5, fig. 3.
- cf. Reference Dulai1993
Salgirella cf. albertii (Oppel); Dulai, p. 30, pl. 1, fig. 2.
- Reference Manceñido2002
Salgirella alberti (Oppel); Manceñido et al., p. 1266, fig. 862 (1).
- cf. Reference Dulai2003
Salgirella cf. alberti (Oppel); Dulai, p. 29, pl. 5, figs. 7–10.
- Reference Vörös and Dulai2007
Salgirella albertii (Oppel); Vörös and Dulai, p. 54, pl. 1, figs. 17, 18.
- Reference Baeza-Carratalá2008
Salgirella albertii (Oppel); Baeza-Carratalá, p. 178, pl. 8, figs. 1–6.
- Reference Siblík and Lobitzer2008
Salgirella albertii (Oppel); Siblík and Lobitzer, p. 66, pl. 1, fig. 2.
- Reference Mandl, Dulai, Schlögl, Siblík, Szabó, Szente and Vörös2010
Salgirella cf. albertii (Oppel); Mandl et al., p. 93, pl. 2, fig. 8; pl. 6, fig. 6.
- Reference Mandl, Dulai, Schlögl, Siblík, Szabó, Szente and Vörös2010
Salgirella albertii (Oppel); Mandl et al., p. 91, pl. 7, fig. 6(9); pl. 9, fig. 5.
- Reference Vörös2014
Salgirella alberti (Oppel); Vörös, p. 22, figs. 24–26.
- Reference Baeza-Carratalá, Dulai and Sandoval2018b
Salgirella alberti (Oppel); Baeza-Carratalá et al., p. 374, fig. 4 (8–10).
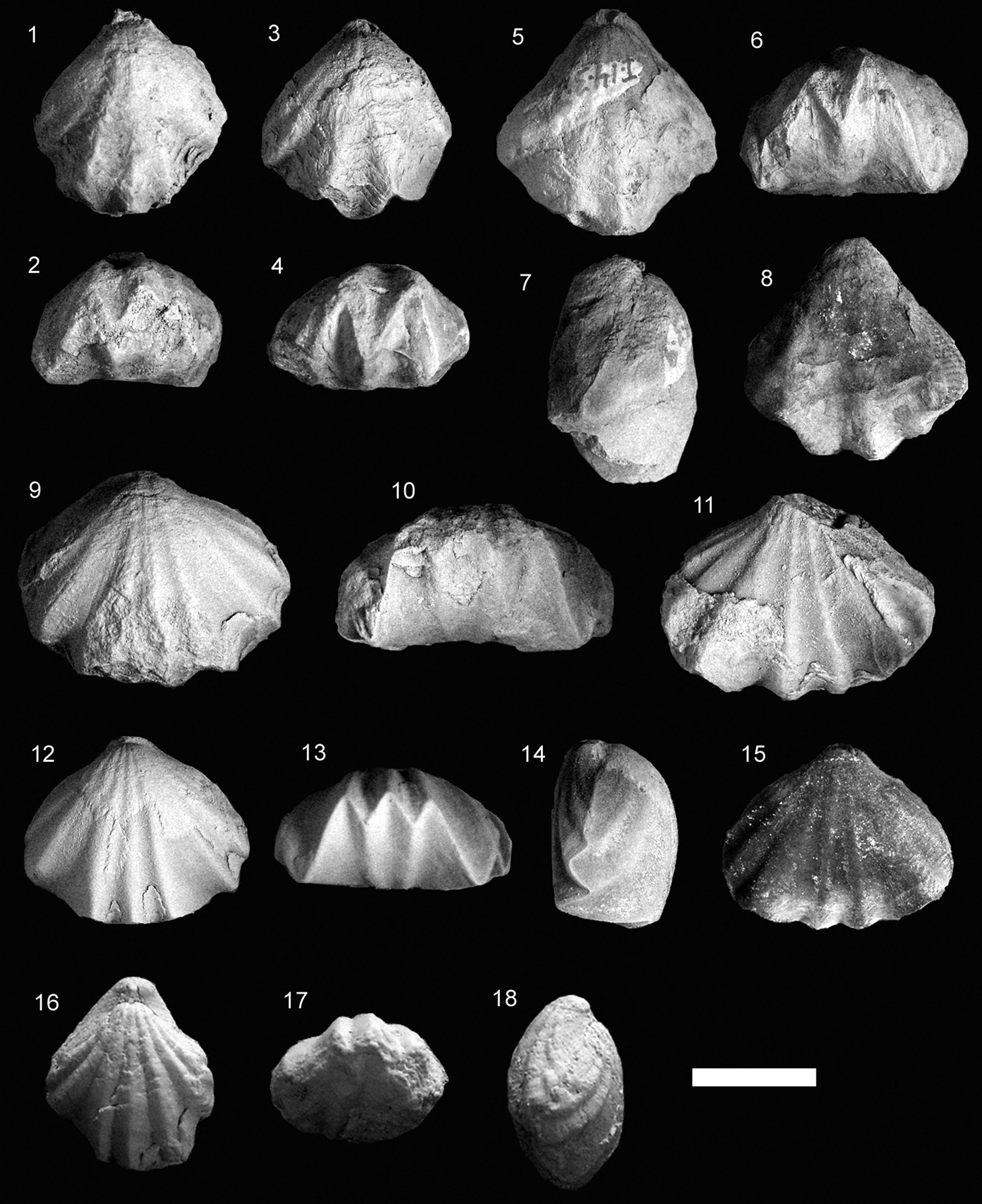
Figure 10. Some representative Early Jurassic specimens of Salgirella alberti (Oppel, Reference Oppel1861) from the Subbetic paleomargin. (1, 2) Specimen LL.al.1 (dorsal and anterior views, respectively); (3, 4) specimen LL.al.2 (dorsal and anterior views, respectively); (1–4) derive from the Pliensbachian-Toarcian transition from Las Losillas (External Subbetic, Murcia); (5–8) specimen I.14.3.9 from the JdC collection (views are ordered consecutively in dorsal, anterior, lateral, and ventral views); (9–11) specimen I.15.3.12 from the JdC collection (dorsal, anterior, and ventral views, respectively); (12–15) specimen O.8.20.T5.1 from the Pliensbachian of Sierra de Quibas (External Subbetic, Murcia); views are ordered consecutively in dorsal, anterior, lateral, and ventral views; (16–18) specimen SGA1.SA1 (dorsal, anterior, and lateral views respectively) from the Sinemurian of Sierra Gorda (Internal Subbetic, Granada). Scale bar = 1 cm.
Holotype
The single specimen figured as Rhynchonella Albertii by Oppel (Reference Oppel1861, pl. 13, fig. 4), stored in the Museum für Naturkunde (Berlin) and collected from the Hierlatz limestones Formation.
Occurrence
This species is frequently reported in the classical literature (e.g., Oppel, Reference Oppel1861; Geyer, Reference Geyer1889; Ormós, Reference Ormós1937). Among the numerous localities where S. alberti is recorded, notable are those from the Sinemurian from the Northern Calcareous Alps (Geyer, Reference Geyer1889; Vörös et al., Reference Vörös, Szabó, Dulai, Szente, Ebli and Lobitzer2003; Siblík and Lobitzer, Reference Siblík and Lobitzer2008; Mandl et al., Reference Mandl, Dulai, Schlögl, Siblík, Szabó, Szente and Vörös2010) and Crimea and Caucasus (Moisseiev, Reference Moisseiev1934; Moisseiev in Berg et al., Reference Berg, Krimholz, Moisseiev, Mjatliuk, Petrova, Pcelincev, Riabinin, Tchernyshev, Sharapova and Yakowlew1947); from the Hettangian–late Sinemurian of the Transdanubian Ranges (Dulai, Reference Dulai1993, Reference Dulai2003; Vörös and Dulai, Reference Vörös and Dulai2007); from the Pliensbachian of the Subbetic (Baeza-Carratalá, Reference Baeza-Carratalá2013; Baeza-Carratalá et al., Reference Baeza-Carratalá, Dulai and Sandoval2018b); from the Pliensbachian of Turkey (Vörös, Reference Vörös2014) or the North African basins (Dareste de la Chavanne, Reference Dareste de la Chavanne1920).
Description
This species was recently exhaustively described and discussed in several western Tethys biochoremas (Dulai, Reference Dulai2003; Vörös and Dulai, Reference Vörös and Dulai2007; Baeza-Carratalá, Reference Baeza-Carratalá2008; Vörös, Reference Vörös2014; Baeza-Carratalá et al., Reference Baeza-Carratalá, Dulai and Sandoval2018b; among others) and well illustrated over the last decades, as can be deduced from the synonymy list, and does not need further detailed systematic external description, but its great intraspecific variability justifies an abridged description of S. alberti in the peri-Iberian paleomargins, thus substantiating our interpretation of this taxon. Betic material of this species corresponds to medium-sized dorsibiconvex shells, with triangular/subpentagonal to pyriform dorsal outline. W/L ratio is rather variable. Maximum width lies on the anterior third of the shell. The beak is broad, strong, and slightly incurved, with a minute pedicle foramen; the beak ridges are short and barely perceivable, and planareas are not well developed. Commissure is straight laterally and uniplicate at anterior margin, with a narrow and subtrapezoidal to arcuate dorsal median fold. Coarsely ribbed shell, with 9–13 triangular ribs (2–4 on the median fold) without bifurcation. There are well-developed flat, smooth, areas just adjacent to the median fold, clearly separating the top of the dorsal fold from the flanks of the shell.
Serial sections of S. alberti (Fig. 11) show a rectangular delthyrial cavity and semicircular umbonal cavities. Pedicle collar is present. Deltidial plates are triangular in cross-section. Dental plates short, initially divergent, then subparallel. Hinge teeth massive and crenulated, as are the sockets. Small denticula visible. A short dorsal median septum and a poorly developed, shallow, wide, U-shaped septalium are discernible. Fused hinge plates flat and subhorizontal. Hamiform crural development, with comma-shaped crural plates (Fig. 11.2, 11.5). Secondary layer of the shell shows an eurinoid pattern, with rhombic/subrectangular cross-sectional outline of the calcite fibers, ~50–60 μm wide and 20–30 μm thick (Fig. 5.4).

Figure 11. Internal structure of Salgirella alberti (Oppel, Reference Oppel1861) from the Pliensbachian (Eastern Prebetic); serial sections orientated with the ventral valve up. (1) Specimen I.12.T26(26).2 in which serial sections were performed; (2) transverse serial sections through the same specimen (distance from the apex in mm); (3–5) photomicrographs of acetate peels from the same specimen: (3) section at 1.10 mm showing dorsal median septum and short septalium; (4) section at 1.90 mm showing the final part of the fused hinge plates; also notice the interlocked dentition and the beginnings of the crural bases; (5) section at 3.30 mm with separated hamiform crura. Scale bars = 1 cm (1), 2 mm (2), and 1 mm (3–5).
Material
Twenty-five articulated specimens in different states of preservation sampled from the Cerro de la Cruz, La Algueda, and Las Losillas outcrops in the External Subbetic; 6 fragmented specimens from the Internal Subbetic (Sierra Gorda); supplemented by 32 specimens from the JdC collection derived from Sierra de la Espada, Sierra de Quibas, Algayat, and Cerro Cruz Algueña.
Remarks
Although variability of this species has been extensively analyzed and discussed by previous authors (Moisseiev in Berg et al., Reference Berg, Krimholz, Moisseiev, Mjatliuk, Petrova, Pcelincev, Riabinin, Tchernyshev, Sharapova and Yakowlew1947; Dulai, Reference Dulai2003; Vörös and Dulai, Reference Vörös and Dulai2007; Baeza-Carratalá, Reference Baeza-Carratalá2008; Mandl et al., Reference Mandl, Dulai, Schlögl, Siblík, Szabó, Szente and Vörös2010; Vörös, Reference Vörös2014; Baeza-Carratalá et al., Reference Baeza-Carratalá, Dulai and Sandoval2018b; among others), short remarks about the Betic material must be emphasized due to the great variability reported in the previous literature. Baeza-Carratalá (Reference Baeza-Carratalá2008) and Baeza-Carratalá et al. (Reference Baeza-Carratalá, Dulai and Sandoval2018b) indicated that the intraspecific variability of this species can vary between two extreme morphotypes: that from Salgir River, with larger and robust beak, fewer and stronger ribs, and narrower outline, referred to by Manceñido et al. (Reference Manceñido, Owen, Dagys and Selden2002) and considered by several previous authors (e.g., Dareste de la Chavanne, Reference Dareste de la Chavanne1920; Baeza-Carratalá, Reference Baeza-Carratalá2008, Reference Baeza-Carratalá, Dulai and Sandoval2018b, part); versus the classical long-established morphotype intended by Oppel (Reference Oppel1861), showing wider outline, pointed beak, and more prominent dorsal fold. The Pliensbachian material in the Subbetic basins shows both extremes of variability. Moreover, the westernmost Subbetic specimens, Pliensbachian in age, have more-pronounced smooth areas developed just adjacent to the median fold than the Sinemurian material from the Internal Subbetic (Baeza-Carratalá et al., Reference Baeza-Carratalá, Dulai and Sandoval2018b).
The costation is a relatively consistent character. Specimens displaying 11 ribs on the entire shell are the most frequent, often with a variable number of ribs located on the median fold (and thus in the ventral sinus, where commonly 2–3 ribs occur). Some other variable features are related to the median dorsal fold (e.g., its outline ranging from higher subtriangular to trapezoidal and lower in shape).
Concerning the internal structure, short dental plates and septum, presence of septalium, and crural development suggest inclusion in the genus Salgirella. The specimen sectioned in this work agrees well with Moisseiev's material (Moisseiev in Berg et al., Reference Berg, Krimholz, Moisseiev, Mjatliuk, Petrova, Pcelincev, Riabinin, Tchernyshev, Sharapova and Yakowlew1947) and with the serial sections depicted by Manceñido et al. (Reference Manceñido, Owen, Dagys and Selden2002). Only minor variations can be detected, such as a shorter dorsal median septum in the Subbetic material than that from Salgir River sectioned by Lobacheva and illustrated by Manceñido et al. (Reference Manceñido, Owen, Dagys and Selden2002) and, in turn, Moisseiev in Berg et al. (Reference Berg, Krimholz, Moisseiev, Mjatliuk, Petrova, Pcelincev, Riabinin, Tchernyshev, Sharapova and Yakowlew1947) showed shorter dental plates than the Subbetic material.
As specified above, the folding pattern of Cirpa briseis is comparable to and usually difficult to distinguish from S. alberti, if we consider that they are commonly recorded together in several western Tethyan basins. In this sense, S. alberti develops wider smooth areas on both flanks of the dorsal median fold and more-spaced ribs, especially noticed in these areas in the corresponding ventral valve sulcus (Fig. 10).
The variety sospirolensis (Uhlig, Reference Uhlig1879) is not conspecific with S. alberti (Oppel), because it differs in having wide and deeper Prionorhynchia-type planareas. The same is applicable to the specimen figured by Principi (Reference Principi1910), which was tentatively assigned by Vörös (1994) to the genus Homoeorhynchia. The very flat dorsal valve and lesser development of contiguous areas to the median fold figured by Fucini (Reference Fucini1895) or Böse and Schlosser (Reference Böse and Schlosser1900) could fit better in the conspecific range of Cirpa briseis. The complex taxonomical combination Rhynchonella n. sp. ind. ex affin. alberti, adopted by Haas (Reference Haas1912), has smooth posterior stage; it was revised as Homoeorhynchia? lubrica by Vörös (Reference Vörös2009), whereas the variety minor (Ormós, Reference Ormós1937) shows densely packed ribs, flatter valves, and wider uniplication.
Apart from the aforementioned frequently misidentified C. briseis-S. alberti, the closest species is Salgirella? magnicostata (Ormós, Reference Ormós1937), but the latter taxon is larger and has a strong ribbing pattern.
Finally, concerning the spelling adopted as valid for this species name, even though Oppel (Reference Oppel1861, p. 546) correctly formed a genitive from the surname Alberti, we agree with Vörös (Reference Vörös2014, p. 22) that the emendation to “alberti” by Geyer (Reference Geyer1889, p. 43, with the mandatory change of Art. 28) corresponds to a prevailing usage (cf., synonym list above), deemed to be a correct original spelling, to be preserved under Art. 33.3.1 of the ICZN (1999).
Salgirella? goicoecheai Baeza-Carratalá, Reference Baeza-Carratalá2011
Figure 12
- Reference Baeza-Carratalá2008
Salgirella? goicoecheai Baeza-Carratalá, p. 185, pl. 9, figs. 3, 4.
- Reference Baeza-Carratalá2011
Salgirella? goicoecheai Baeza-Carratalá, p. 350, fig. 2 (3, 4).

Figure 12. Endemic Pliensbachian species Salgirella? goicoecheai Baeza-Carratalá, Reference Baeza-Carratalá2011, from the Subbetic paleomargin. (1–3) Specimen O.7.22.2 (holotype) from the Moleta de Togores outcrop (JdC collection); views are ordered consecutively in dorsal, anterior, and lateral views; (4–6) specimen O.7.22.1 from the Moleta de Togores outcrop (JdC collection); views are ordered consecutively in dorsal, anterior, and lateral views; (7–10) specimen CI4550 derived from the Pliensbachian of Cerro de La Cruz-1 (Peiró collection, MUPE); views are ordered consecutively in dorsal, anterior, lateral, and ventral views; (11) fragmented specimen CCA.10.Sgo.CH (anterior view) from the Pliensbachian of Cerro de la Cruz-1; (12–14) specimen CI4548 derived from the Pliensbachian of Cerro de La Cruz-1 (Peiró collection, MUPE); views are ordered consecutively in dorsal, anterior, and ventral views. Scale bar = 1 cm.
Holotype
Specimen O.7.22.2 selected by Baeza-Carratalá (Reference Baeza-Carratalá2011, fig. 2.3) and figured herein (Fig. 12.1–12.3), deposited at the Paleontological Museum of Murcia, Spain (JdC collection). Middle–late Pliensbachian from the Cerro de La Cruz of La Romana (Alicante, Spain). Upper member of the Gavilán Formation.
Occurrence
This species is endemic to the Eastern Subbetic Domain, and it is recorded from the late Pliensbachian.
Description
This is a large-sized multicostate rhynchonellide that was erected as a new species endemic to the Subbetic area by Baeza-Carratalá (Reference Baeza-Carratalá2011). It is pentagonal and wider than long in dorsal outline. The beak is wide and suberect and the dorsal folding pattern consists of a trapezoidal and wide uniplication corresponding with a well-marked ventral sulcus. Ribbing pattern consists of 16–23 densely packed, acute, and triangular ribs (5–8 on the dorsal fold) without bifurcation.
Material
Six articulated specimens sampled from Cerro de La Cruz-1 (1 DCTMA; 2 Peiró collection, MUPE) and Cerro de La Cruz-2 (3 DCTMA), supplemented by 16 articulated specimens from the JdC Collection (Moleta de Togores outcrop).
Remarks
Because the internal structure remains unknown owing to inadequate material for making serial sections, the beak features, folding pattern, presence of wide smooth areas separating the dorsal median fold from both flanks, and the very remarkable ventral sulcus have been considered as discriminant criteria for the provisionally tentative assignation to the genus Salgirella. Additionally, partial sectioning of fragmentary material has not conclusively revealed clear information about the pattern of the fibers concerning the shell microstructure.
This taxon can be unambiguously distinguished from its apparent congeneric relatives due to the consistently greater size, the markedly trapezoidal dorsal fold, and the numerous and more densely packed ribs. Comparable forms can be found only in the widely interpreted Rhynchonella gr. variabilis, which is considered a nomen dubium and should be revised.
Results
Morphometric analysis carried out on the dataset allows for establishment of a systematic scheme for the Pliensbachian–Toarcian peri-Iberian Wellerelloidea. Principal Component Analysis (PCA) and Canonical Variate Analysis (CVA) provide a better understanding of the morphological variability within the peri-Iberian Wellerelloidea and the potential relationships in this group (Fig. 13). Among all dimensional features considered, the main biometric parameters (L, W, T), number of ribs (R, Rf), data related to smooth areas alongside the median fold (dpi, dr), and the height and width of the fold (fh, wt, wb) are the more significant criteria to perform these analyses.

Figure 13. Morphometrical scatter plots of the studied wellerelloid specimens. (1) Morphospaces defined by the two main axes of the Principal Component Analysis (PCA) applied to the Cirpinae representatives of the peri-Iberian basins. (2) Morphospaces defined by the two main discriminant axes of the Canonical Variate Analysis (CVA) applied to the same samples. In both analyses, scores are clustered in terms of taxonomical discrimination showing a diverse occupation of the morphospace. Dimensional vectors have been superimposed on the PCA and CVA values showing the main discriminating factors. Abbreviations as in Fig. 2.
The first two principal components obtained by the PCA (PC1 and PC2 axes) explain >88% of the variance within the data (Fig. 13.1), and accordingly have been considered as representing the variability of the group. If the dimensional vectors are superimposed on the PCA scatter plot (Fig. 13.1), several patterns emerge, as discussed below.
Cirpa briseis is located in a central position of the scatter plot, dominating the principal morphospace of the PCA, showing a great range of intraspecific variability related to the main biometric parameters (L, T, W), thus revealing a size-related distribution, but also differing from the other species of Cirpa by the folding pattern, acquiring higher-folded morphotypes (indicated for more negative values along PC2) and, in turn, showing fewer ribs than their congeneric individuals analyzed. Among the remaining species of Cirpa, the main discriminant factor along the PC2 axis is the ribbing pattern and, consequently, the number of ribs on the median fold (R, Rf), as shown in their distribution along the PC2 axis, because samples with more ribs score more positive values along this axis, progressively increasing along the series C. lucentina-C. planifrons-C. fallax (Fig. 13.1). Secondary discriminant factors in this series are the biometric values (e.g., representing Cirpa cf. C. slovenica and C. fallax) and the extreme dimensional forms of Cirpa with costation as the principal discriminant factor and wider-expanded shape than C. lucentina n. sp., C. subcostellata, and C. planifrons. Importance of the ribbing pattern and its densely packed arrangement as diagnostic criteria in discrimination of the studied species of Cirpa is apparent (Fig. 14).

Figure 14. Bivariate plot of width (W) vs. number of ribs (R) on the studied specimens. The graph shows a positive correlation of the number of ribs with the width in each species of Cirpa, as expected. Notice that there is not overlapping among different groups, validating the ribbing density as an appropriate diagnostic criterion, except for C. briseis and S. alberti, where some other criteria considered in the PCA and CVA analyses are involved.
The discriminant CVA analysis (Fig. 13.2) reinforces the influence of C. subcostellata, C. briseis, and C. lucentina n. sp. as the representative stock of the group, from which species assigned to Salgirella differ. Thus, S. alberti and S.? goicoecheai are located in the left half of the discriminant axis 1 and the younger species of Cirpa (C. fallax) occupies the right side of this axis. The scatter plot of variable loadings in the CVA shows ribbing density and folding pattern as the main discriminating features (Fig. 13.2). Discriminant analysis also reinforces the accuracy of the systematic splitting between the genera Cirpa and Salgirella based on differentiation of the median dorsal fold since Salgirella reaches higher values along the dimensional vector related to the development of smooth areas separating the median dorsal fold from the flanks of the shell (dpi, dr), which in turn results in a more pronounced median ventral sulcus.
The results of these morphometric analyses alongside details of the internal structures conducted in this work reveal useful diagnostic criteria to distinguish Cirpa and Salgirella, considered as possibly synonymous by some previous authors, and complement the work of previous authors (Moisseiev in Berg, Reference Berg, Krimholz, Moisseiev, Mjatliuk, Petrova, Pcelincev, Riabinin, Tchernyshev, Sharapova and Yakowlew1947; Ager, Reference Ager1958, Reference Ager1959, Reference Ager, Adams and Ager1967; Siblík, Reference Siblík1967, Reference Siblík1993a; Tchoumatchenko, Reference Tchoumatchenco1989; Böhm et al., Reference Böhm, Ebli, Krystin, Lobitzer, Rakús and Siblík1999; Alméras and Fauré, Reference Alméras and Fauré2000; Manceñido et al., Reference Manceñido, Owen, Dagys and Selden2002; Dulai, Reference Dulai2003; Baeza-Carratalá, Reference Baeza-Carratalá2008; Siblík and Lobitzer, Reference Siblík and Lobitzer2008; Vörös, Reference Vörös2014; among others).
Discussion
Lower Jurassic wellerelloids from the Iberian paleomargins
The basal stock of the wellerelloid cirpines in the Iberian paleomargins is integrated into the genus Calcirhynchia. The oldest occurrences of this genus were attributed to C. calcaria Buckman, Reference Buckman1918, and C. calcicosta (Quenstedt, Reference Quenstedt1852) from the early Sinemurian (Bucklandi–Semicostatum chronozones) in the Internal Subbetic of Sierra Harana (Pérez-López et al., Reference Pérez-López, Martín-Algarra, Alméras and Foucault1993). Several species of Calcirhynchia proliferated in the brachiopod communities during the Sinemurian of the Internal and External Subbetic areas; thus, in the early–late Sinemurian of Sierra Gorda (Turneri–Raricostatum chronozones), Calcirhynchia hungarica (Böckh, Reference Böckh1874) and C. aff. C. rectimarginata (Vecchia, Reference Vecchia1945) were recorded together with the first representatives of the genera Cirpa and Salgirella (C. subcostellata and S. alberti) (Fig. 15). Finally, in the late Sinemurian–earliest Pliensbachian (Raricostatum–Aenigmaticum chronozones) from the easternmost Subbetic domain, C. plicatissima (together with Prionorhynchia regia [Rothpletz, Reference Rothpletz1886]) dominated the multicostate rhynchonellide stock (Baeza-Carratalá, Reference Baeza-Carratalá2013; Baeza-Carratalá et al., Reference Baeza-Carratalá, Giannetti, Tent-Manclús and García Joral2014) within a highly diversified assemblage.

Figure 15. Biostratigraphical distribution of the Lower Jurassic species attributed to Cirpa and Salgirella in the western Tethys. Stippled bars denote taxa recorded in the peri-Iberian paleomargins. Sources cited in the text. ETMEE in the early Serpentinum Chronozone indicates the extinction boundary, as the maximum impact of the Toarcian crisis.
A considerable amount of work remains to be done in the taxonomy of this basal multicostate stock, as previous authors considered (e.g., Tomašových, Reference Tomašových2006; Vörös, Reference Vörös2009). This is especially apparent in species of the genus Calcirhynchia, as also observed in the Subbetic by the occasional adoption of open taxonomic nomenclature (e.g., C. aff. C. rectimarginata; Baeza-Carratalá et al., Reference Baeza-Carratalá, Dulai and Sandoval2018b) and the possible over-lumped attribution to C. plicatissima. This last taxon is a catch-all species reported widely in the western Tethys during the Sinemurian–Pliensbachian interval. Recent revisions to the taxonomic criteria of this group, such as the presence of planareas, interareas, and internal structure (Tomašových, Reference Tomašových2006; Vörös, Reference Vörös2009), should be borne in mind to split several attributions to C. plicatissima into different species and genera, but this is beyond the scope of this study, mainly focused on their Pliensbachian–Toarcian counterparts and their possible evolution around the ETMEE.
Pliensbachian brachiopod assemblages underwent a notable turnover in the peri-Iberian platforms (at least in their Mediterranean margins) with respect to the previous Sinemurian–Pliensbachian transition communities (Baeza-Carratalá, Reference Baeza-Carratalá2013). Regarding cirpines, the prolific record of Calcirhynchia in the Sinemurian gave rise to a phyletic(?) within-family turnover. Thus, Calcirhynchia is not recorded again during Pliensbachian–Toarcian times and Wellerellidae is well represented by several species of the genus Cirpa (Fig. 15), with the conspicuous record of the pervasive Cirpa briseis (in the Subbetic and transitional Prebetic/Subbetic zones) and, to a lesser extent, by C. planifrons and C. cf. C. slovenica, together with two endemic species ascribed to the Betic Domain (Cirpa lucentina n. sp. and Salgirella? goicoecheai). Only Salgirella alberti overtook the renewal of ecospaces as a result of the compartmentalization of the seafloor (Sandy, Reference Sandy1995; cf., Vörös and Dulai, Reference Vörös and Dulai2007; Baeza-Carratalá, Reference Baeza-Carratalá2013) that occurred in the Sinemurian-Pliensbachian transition (Fig. 15). Nevertheless, the occurrence of this species reveals a biostratigraphical gap from the middle Sinemurian to the late Pliensbachian in the peri-Iberian platforms (Fig. 15) while it was spread across several Pontic and Mediterranean basins, suggesting either a migration event or sampling bias in the Betic basins.
With respect to the peri-Iberian endemic cirpines, Cirpa lucentina n. sp. was previously attributed to Cirpa aff. C. latifrons, as discussed above, left in open nomenclature but clearly belonging to the genus Cirpa (Baeza-Carratalá, Reference Baeza-Carratalá2013, Baeza-Carratalá et al., Reference Baeza-Carratalá, García Joral and Tent-Manclús2016b). The comprehensive work by Tomašových (Reference Tomašových2006) on the Hettangian–Sinemurian Rhynchonella latifrons from the Western Carpathians and topotypic material of Rhynchonella latifrons Geyer, Reference Geyer1889, from the Alps, suggested erection of the genus Jakubirhynchia, supporting the separation of a new Cirpa constituent for the Subbetic representatives with younger biostratigraphic distribution.
Rodrigo (Reference Rodrigo2011) reported a few specimens of Cirpa cf. C. briseis in the northern part of the Iberian Cordillera, but the external features, as well as the lack of data on the internal structure, make this attribution within the genus Cirpa uncertain. Thus, we consider it preferable to exclude such a single record from the analysis, awaiting further accurate data. In the northern basins around the Iberian Massif, C. briseis is recorded in the Asturian basin (Comas-Rengifo and Goy, Reference Comas-Rengifo and Goy2010) in the Davoei-Margaritatus zones of the Pliensbachian.
Finally, Cirpa fallax is recorded in the Polymorphum Zone of the lower Toarcian from the Lusitanian Basin (aff., Alméras, Reference Alméras, Copper and Jin1996; Comas-Rengifo et al., Reference Comas-Rengifo, Duarte, García Joral and Goy2013, Reference Comas-Rengifo, Duarte, Félix, García Joral, Goy and Rocha2015), associated with the koninckinid fauna, representing the last Wellerellidae prior to their extinction in the ETMEE interval (Fig. 15). This last stock of cirpines could have reached northern basins, such as Normandy where Rhynchonella fallax Deslongchamps, Reference Deslongchamps1862, was formerly recorded in the early Toarcian (see Alméras, Reference Alméras, Copper and Jin1996, p. 8).
Paleobiogeographic distribution in the western Tethys and implications around the ETMEE
The Lower Jurassic record of Cirpa and Salgirella shows a widespread paleogeographic distribution throughout all the western Tethys biochoremas (Fig. 16), but by evaluating the biostratigraphic occurrences of the different taxa in each Tethyan basin, the ancestral source, radiation pattern, and progression of the diversification of these cirpines can be understood.

Figure 16. Paleobiogeographical distribution of the last wellerelloid representatives. (1) Paleomap around the ETMEE (182 Ma), showing the location of the western Tethys localities in a global context. (2) Paleogeographical distribution of Cirpa and Salgirella in the Tethys Ocean for the Sinemurian–Toarcian interval to better understanding the origin, diversification, and radiation of both genera in the western Tethyan basins (all the sources cited in the text). Occurrences plotted on an Early Jurassic paleomap, slightly modified after Bassoullet et al. (Reference Bassoullet, Elmi, Poisson, Ricou, Cecca, Bellión, Giraud, Baudin, Decourt, Ricou and Vrielynck1993).
Salgirella initially was restricted to the most intra-Mediterranean Subprovince (sensu Vörös, Reference Vörös2016; Vörös et al., Reference Vörös, Kocsis and Pálfy2019). Salgirella alberti, along with the possible congeneric Sinemurian S.? magnicostata represent the earliest occurrence in this region (Fig. 16), mainly from the Northern Calcareous Alps and the Transdanubian Ranges (e.g., Geyer, Reference Geyer1889; Vörös et al., Reference Vörös, Szabó, Dulai, Szente, Ebli and Lobitzer2003; Vörös and Dulai, Reference Vörös and Dulai2007; Siblík and Lobitzer, Reference Siblík and Lobitzer2008; Mandl et al., Reference Mandl, Dulai, Schlögl, Siblík, Szabó, Szente and Vörös2010). In this core of the Mediterranean region, Salgirella would be a constituent of the multicostate rhynchonellide stock, representing the brachiopod faunal recovery in the aftermath of the end-Triassic mass extinction event, since several tentative records were attributed to Salgirella cf. S. alberti in the Hettangian-Sinemurian from the Transdanudian Ranges (Dulai, Reference Dulai1998, Reference Dulai2003; Vörös and Dulai, Reference Vörös and Dulai2007). As early as the middle-late Sinemurian, the distribution of the type species of the genus, S. alberti, had spread to the peri-Mediterranean Subprovince, as reported in the Internal Subbetic by Baeza-Carratalá et al. (Reference Baeza-Carratalá, Dulai and Sandoval2018b).
The central pivotal position of the Alpine/Hungarian Mediterranean ancestral source area enabled east- and westward migration of Salgirella in the Pliensbachian, as denoted by the eastern occurrences of S. alberti in Crimea and Caucasus (Moisseiev, Reference Moisseiev1936; Ruban and Vörös, Reference Ruban and Vörös2015) and Turkey (Vörös, Reference Vörös2014) from the Pontic Bioprovince (Vörös et al., Reference Vörös, Kocsis and Pálfy2019) and, in turn, by those from the Tell (Dareste de la Chavanne, Reference Dareste de la Chavanne1920) in the westernmost African margins (Atlas Subprovince sensu Vörös et al., Reference Vörös, Kocsis and Pálfy2019). The westward dispersal of Salgirella may have been facilitated by several stepping-point epioceanic areas (Fig. 16) such as the Trento platform (Böse and Schlosser, Reference Böse and Schlosser1900), Toscana (Fucini, Reference Fucini1895), and Umbria (Principi, Reference Principi1910), but some of these attributions are ambiguous, as indicated above in the synonymy list of S. alberti. The radiation of Salgirella never reached the epicontinental platforms of the broad Euro-Boreal Bioprovince (Fig. 16). The diversification and migration of this genus always occurred in epioceanic habitats.
A comparable initial homeland is inferred for the genus Cirpa. The intra-Mediterranean records from the Transdanubian Ranges and Northern Calcareous Alps are the more ancient occurrences of the genus worldwide (Figs. 15, 16), being typified by C. planifrons in the Hettangian–Sinemurian from Hungary (Ormós, Reference Ormós1937; Dulai, Reference Dulai2003; Vörös and Dulai, Reference Vörös and Dulai2007) and the Alps (Siblík, Reference Siblík1993a; Böhm et al., Reference Böhm, Ebli, Krystin, Lobitzer, Rakús and Siblík1999; Mandl et al., Reference Mandl, Dulai, Schlögl, Siblík, Szabó, Szente and Vörös2010). This species shows a continuous record throughout the Sinemurian and is coeval with C. subcostellata and C.? subfurcillata (Böse, Reference Böse1897) in both intra-Mediterranean areas (Böse, Reference Böse1897; Vörös and Dulai, Reference Vörös and Dulai2007; Mandl et al., Reference Mandl, Dulai, Schlögl, Siblík, Szabó, Szente and Vörös2010), with both latter species reaching the Pliensbachian age (Vörös and Dulai, Reference Vörös and Dulai2007; Vörös, Reference Vörös2009). Hence, one can deduce that the first diversification episode of Cirpa took place in its native area, because, apart from the three mentioned species, C. fronto also occurred in the late Sinemurian of the Transdanubian Ranges (Vörös and Dulai, Reference Vörös and Dulai2007).
An initial westward radiation episode of the genus Cirpa occurred in the Sinemurian, since C. subcostellata is recorded in the middle-late Sinemurian from the peri-Mediterranean Subprovince (Betics; Baeza-Carratalá et al., Reference Baeza-Carratalá, Dulai and Sandoval2018b) and C. fronto, C. planifrons, and C. subcostellata occurred in the late Sinemurian at the African margins (Alméras et al., Reference Alméras, Elmi and Fauré2007). The timing of the eastward migration of Cirpa in the Sinemurian is unclear because the record of C. kiragliae Ager, Reference Ager1959, in Turkey is referred to the late Sinemurian–early Pliensbachian (Ager, Reference Ager1959; Vörös, Reference Vörös2014).
In the Pliensbachian, both the pervasive C. briseis and C. fronto rapidly colonized the Western Tethys Ocean as a whole, even over the epicontinental seas of the Euro-Boreal Province, consequently leading to the maximum diversity and expansion of Cirpa. The worldwide Pliensbachian dispersal of Cirpa briseis reached regions as paleogeographically diverse as the Lombardian Alps (Parona, Reference Parona1884; Sacchi Vialli and Cantaluppi, Reference Sacchi Vialli and Cantaluppi1967); western Sicily (Gemmellaro, Reference Gemmellaro1874; Di Stefano, Reference Di Stefano1891); Trento platform (Böse and Schlosser, Reference Böse and Schlosser1900; Benigni, Reference Benigni1978); Pyrenees?, Aquitaine Basin, and the French Central Massif (Alméras and Elmi, Reference Alméras and Elmi1987; Alméras and Fauré, Reference Alméras and Fauré2000, Reference Alméras and Fauré2013); Betic Ranges (Baeza-Carratalá, Reference Baeza-Carratalá2013; Baeza-Carratalá et al., Reference Baeza-Carratalá, García Joral and Tent-Manclús2016b); the African Tell (Dareste de la Chavanne, Reference Dareste de la Chavanne1920) and Algeria (Alméras et al., Reference Alméras, Elmi and Fauré2007); the Northern Alps (Vörös et al., Reference Vörös, Szabó, Dulai, Szente, Ebli and Lobitzer2003; Mandl et al., Reference Mandl, Dulai, Schlögl, Siblík, Szabó, Szente and Vörös2010); the Asturian basin (Comas-Rengifo and Goy, Reference Comas-Rengifo and Goy2010); Turkey (Vörös and Kandemir, Reference Vörös and Kandemir2011) or Mecsek (Vörös, Reference Vörös1997); among many others.
In this diversity maximum, the western stock is represented (apart from C. briseis) by several Mediterranean taxa tentatively attributed to Cirpa, such as C.? delottoi (Dal Piaz, Reference Dal Piaz1907) near the Trento Platform, C.? latissima (Fucini, Reference Fucini1895) in the Toscana region, C.? eleutheria (Di Stefano, Reference Di Stefano1891) and C.? iphimedia (Di Stefano, Reference Di Stefano1891) in W-Sicily, and now C. lucentina n. sp. in the Betic Ranges. The westernmost records are found in the Atlas Subprovince with the cosmopolitan C. briseis and C. fronto together with C. subcostellata (Alméras et al., Reference Alméras, Elmi and Fauré2007).
In the Western Carpathians, closer to the intra-Mediterranean core, Pliensbachian Cirpa is represented by C. slovenica Siblík, Reference Siblík1967, whereas the Pontide diversification eastwards resulted in C. langi and C. borissiaki (Moisseiev, Reference Moisseiev1934) occurring in Bulgaria, Crimea, North-Caucasus, and Turkey (e.g., Moisseiev, Reference Moisseiev1934; Tchoumatchenco, Reference Tchoumatchenco1989; Vörös and Kandemir, Reference Vörös and Kandemir2011; Vörös, Reference Vörös2014; Ruban and Vörös, Reference Ruban and Vörös2015), plus C. alkayae Vörös, Reference Vörös2014, and C. kiragliae (Ager, Reference Ager1959; Vörös, Reference Vörös2014) in Turkey.
The Euro-Boreal diversification involved C. briseis, C. langi (Spinatum Zone from SW-England basins; Ager, Reference Ager1958), and presumably C.? minor (Rau, Reference Rau1905) and C.? major (Rau, Reference Rau1905) in SW-Germany.
In the peri-Iberian platforms system, during the Pliensbachian maximum dispersal, the absence of cirpines is noteworthy in the widely documented prolific brachiopod assemblages from the Iberian Ranges (Fig. 16). Actually, cirpines colonized the Mediterranean South-Iberian (Betic Ranges) and the proto-Atlantic (Portugal and Asturian basins) paleomargins, either via the Lusitanian seaway or, more likely, via the epicontinental French-Pyrenean basins.
In this Pliensbachian diversity peak, expanded migration of Cirpa representatives could have occurred through the Hispanic Corridor, as noted by Manceñido (Reference Manceñido2002). Thus, Andean and Australasian occurrences of Cirpa were recorded in the late Pliensbachian somewhat later than the Tethyan occurrences (Manceñido, Reference Manceñido2002), such as Cirpa seranensis (Wanner and Knipscheer, Reference Wanner and Knipscheer1951) from East Seram (Manceñido and Dagys, Reference Manceñido, Dagys and Westermann1992, and references therein) or Cirpa sp. (Pliensbachian–Early Toarcian) from the Neuquen Basin (Manceñido, Reference Manceñido1990). On the other hand, Manceñido et al. (Reference Manceñido, Owen, Dagys and Selden2002) regard some species assigned to Vincentirhynchia from New Caledonia and New Zealand (MacFarlan, Reference MacFarlan1992) as possible constituents of the genus Cirpa. In this sense, even though some external features of V. uitoeensis (MacFarlan, Reference MacFarlan1992) can fit into the generic concept of Cirpa herein considered, the broader and shallower fold (even rectimarginate in some other species), the presence of divided hinge plates, and the well-defined median septum supported by a deep septalium raise questions in these latest Triassic–Early Jurassic records.
The youngest occurrence of the genus is marked by Cirpa fallax (Toarcian, Polymorphum Zone) from the Lusitanian Basin (cf., Alméras, Reference Alméras, Copper and Jin1996; Comas-Rengifo et al., Reference Comas-Rengifo, Duarte, García Joral and Goy2013, Reference Comas-Rengifo, Duarte, Félix, García Joral, Goy and Rocha2015) and Normandy (Deslongchamps, Reference Deslongchamps1862). The report of Rh. fallax (Rudirhynchia fallax as formerly suggested by Ager, Reference Ager1958, Reference Ager1962) in the Jamesoni Zone from the British basins, turned out to be a species of Prionorhynchia (Ager, Reference Ager, Adams and Ager1967; Alméras, Reference Alméras, Copper and Jin1996). On the other hand, the presence of rimmed foramen and a clear, even bifurcate, dorsal median septum described in Cirpa fromontae Craig, Reference Craig2002, from the early Bajocian of Western Australia (Craig, Reference Craig2002) would require a different generic attribution.
Substantiating the paleobiogeographical evidence, the Mediterranean origin of both Salgirella and Cirpa can be ascertained, but while the diversification of Salgirella took place in epioceanic habitats, speciation of Cirpa also occurred in epicontinental habitats, colonizing the Euro-Boreal Bioprovince, until their extinction in the ETMEE, just prior to the onset of the hyperthermal warming occurred in the basal Serpentinum Chronozone (García Joral et al., Reference García Joral, Gómez and Goy2011; Gómez and Goy, Reference Gómez and Goy2011; Baeza-Carratalá et al., Reference Baeza-Carratalá, García Joral, Goy and Tent-Manclús2018a; among many others).
Contribution to the phyletic relationship of wellerelloids prior to and after the ETMEE
The integrated internal structure/morphometrical external analyses performed on the peri-Iberian Pliensbachian cirpines and their distribution across the western Tethys allow us to evoke previous phyletic schemes in this group in the light of the new taxonomic data.
The progressive succession of the evolutionary lineage Calcirhynchia → Cirpa/Salgirella in the Early Jurassic seems apparent. Among the cirpine representatives, the mainly Hettangian–Sinemurian Calcirhynchia (even though some of them also reached the Pliensbachian, mostly in the Mediterranean Province; e.g., C. hungarica, C.? zugmayeri [Gemmellaro, Reference Gemmellaro1874], or C. ex gr. plicatissima) were replaced by species of Cirpa and Salgirella in the Pliensbachian–early Toarcian, prior to the definitive extinction of Wellerelloidea in the ETMEE.
Baeza-Carratalá and García Joral (Reference Baeza-Carratalá and García Joral2020) inferred a relationship between some structural features and resilience of several groups of rhynchonellides around crisis events. The eurinoid/ribbed/septifal rhynchonellide morphogroup, with cirpines standing among their constituents, typified a resilient pattern in epicontinental habitats. In fact, Cirpa fallax is one of the very few large and multicostate rhynchonellides that reached levels preceding the ETMEE in the western Tethys basins (García Joral et al., Reference García Joral, Baeza-Carratalá and Goy2018).
At this point, one can expect that environmental perturbations around the ETMEE entailed extinction of multicostate rhynchonellides with similar internal structure and external features. However, this structural pattern persisted through the early Toarcian crisis through the genus Pseudogibbirhynchia, which is one of the typical representatives of the often-diminished brachiopod assemblages of the late Toarcian–Aalenian from epicontinental areas (Alméras, Reference Alméras, Copper and Jin1996; Andrade, Reference Andrade2006; García Joral and Goy, Reference García Joral and Goy2009; Andrade et al., Reference Andrade, Duarte, García Joral, Goy and Henriques2016). In fact, a plausible connection between cirpines and the genus Pseudogibbirhynchia may be proposed. The phyletic relationship between Cirpa and Pseudogibbirhynchia goes back for a long time and was formerly envisaged by Ager (Reference Ager1962, p. 110), who suggested that Cirpa was very probably the direct ancestor of Pseudogibbirhynchia. Ager (Reference Ager1962) compared several species within both genera, remarking on the similarity of the crural architecture and the presence of double deltidial plates. Alméras (Reference Alméras, Copper and Jin1996) undertook revision of the Portuguese Pseudogibbirhynchia, also alluding to the similarity between the external and internal structure of both genera. Actually, Pseudogibbirhynchia and Cirpa were formerly assigned to the Family Wellerellidae, Subfamily Cirpinae, by several authors (e.g., Ager, Reference Ager and Moore1965; Rousselle, Reference Rousselle1973; Alméras, Reference Alméras, Copper and Jin1996; Alméras et al., Reference Alméras, Elmi and Fauré2007, Reference Alméras, Cougnon, Guibbert and Fauré2014; among others), and subsequently separated, even at the Superfamily level (Cirpa = Wellerelloidea; Pseudogibbirhynchia = Pugnacoidea). This systematic question was outlined again by Baeza-Carratalá and García Joral (Reference Baeza-Carratalá and García Joral2020) who, when analyzing the rhynchonellide morphogroups around critical post-Paleozoic events, clustered several ribbed Basiliolidae such as Pseudogibbirhynchia together with wellerelloids such as Cirpa, Salgirella, or Calcirhynchia on the basis of their crural development, costation, and microstructure of the shell. Certainly, except for the presence of fused hinge plates in Cirpa and particular differences in the beak features, Pseudogibirhynchia and Cirpa share most of their generic diagnostic criteria, such as the strengthened deltidial plates (even double in Cirpa), hamiform crural development, eurinoid microstructure of the secondary layer of the shell, short to absent median septum, uniplicate anterior margin, and multicostate and often bifurcate ribbed valves.
Bearing in mind the morphotype of the last representatives of the genus Cirpa, typified by Cirpa fallax, a possible connection between this taxon and the post-ETMEE stock of Pseudogibbirhynchia can be postulated. In fact, Alméras (Reference Alméras, Copper and Jin1996), in the absence of information on internal shell structures, considered the species Rh. fallax (Deslongchamps) from Portugal as belonging to Pseudogibbirhynchia. As a matter of fact, by analyzing the intraspecific variability of the specimens depicted as Pseudogibirrynchia fallax by Alméras (Reference Alméras, Copper and Jin1996), they properly fit in the concept of the post-ETMEE stock of Pseudogibbirhynchia (e.g., flattened outline, more densely packed ribs than the typical pattern of Cirpa, occasionally bifurcate ribs), but have a higher fold than the standard morphotypes of Pseudogibbirhynchia. However, serial sections of the Portuguese specimens from the same region (Fig. 7) clearly revealed fused hinge plates, absence of dorsal median septum and septalium, and double deltidial plates, among other characters, which led Comas-Rengifo et al. (Reference Comas-Rengifo, Duarte, García Joral and Goy2013, Reference Comas-Rengifo, Duarte, Félix, García Joral, Goy and Rocha2015) to place Rh. fallax in the genus Cirpa. It can be pointed out that Cirpa fallax represents the last recorded occurrence of the genus Cirpa prior to the ETMEE, and due to their evident internal and external analogy with several post-extinction Pseudogibbirhynchia species (e.g., P. jurensis [Quenstedt, Reference Quenstedt1858], P. bothenhamptonensis [Walker, Reference Walker1892], P. mutans [Rothpletz, Reference Rothpletz1886]), one can postulate that C. fallax represented, either the link between the pre-extinction Cirpa and the post-ETMEE Pseudogibbirhynchia stock well developed in the repopulation interval in the peri-Iberian basins (García Joral et al., Reference García Joral, Gómez and Goy2011), or a Lazarus taxon capable of reappearing after the collapse habitats for the rhynchonellides, as a result of the hyperthermal maximum that occurred in the extinction boundary (García Joral et al., Reference García Joral, Gómez and Goy2011, Reference García Joral, Baeza-Carratalá and Goy2018; Baeza-Carratalá et al., Reference Baeza-Carratalá, Reolid and García Joral2017, Reference Baeza-Carratalá, García Joral, Goy and Tent-Manclús2018a).
The combination of some internal characters (hinge plate development, straightened deltidial plates, etc.) could lead to misinterpretations in the unusual attributions to the genus Cirpa in the Bajocian from remote paleobiogeographic domains, such as Cirpa fromontae from Western Australia (Craig, Reference Craig2002) with a patent (even bifurcate) dorsal median septum, or Cirpa himalaica (Jin et al., Reference Jin, Rong and Sun1976) from China, which proved to be a new genus after a detailed systematic revision (Shi and Yang, Reference Shi and Yang1990).
In contrast with this phyletic hypothesis, it should be taken into account that the earliest record so far known of the genus Pseudogibbirhynchia is P. globosa Siblík, Reference Siblík1967, in the uppermost Pliensbachian from Slovakia, and the first occurrences of the type species P. moorei reported in Somerset (e.g., Ager, Reference Ager1962) in the “Junction Beds” (Spinatum–Tenuicostatum zones), younger than the koninckinid fauna. Pseudogibbirhynchia moorei from Dorset (Ager, Reference Ager1962) has the typical cirpine internal structure, even the double deltidial plates (Ager, Reference Ager1962, text-fig. 67), similar to C. fallax. Some pre-ETMEE records have been attributed to Pseudogibbirhynchia, such as the upper Pliensbachian Pseudogibbirhinchia sp. Benigni, Reference Benigni1978, from the Trento platform, but its coarse and sparsely packed ribbing pattern would suggest affinities with other genera. Similarly, the upper Sinemurian Rh. moghrabiensis Dubar, Reference Dubar1942, from Morocco, shows similar internal structure and costation, but the great convexity of both valves and the rounded outline of the anterior margin are not conclusive, because these features are not representative for the genus Pseudogibbirhynchia.
Thus, Pseudogibbirhynchia, well established in the recovery interval in the upper Toarcian–Aalenian from Spain, Portugal, the Atlas, and the Alps (Rousselle, Reference Rousselle1973; García Joral, Reference García Joral, Pálfy and Vörös1993; Andrade, Reference Andrade2006; García Joral and Goy, Reference García Joral and Goy2009; Andrade et al., Reference Andrade, Duarte, García Joral, Goy and Henriques2016), could represent continuity of the eurinoid/septifal/ribbed stock, post-ETMEE, and therefore the close evolutionary relationship between Cirpa and Pseudogibbirhynchia seems to be feasible.
Conclusions
Superfamily Wellerelloidea represents a long-ranging rhynchonellide clade inhabiting different Paleozoic and Mesozoic habitats. The last representatives of this clade (Subfamily Cirpinae) in the Early Jurassic typify a pervasive pattern of colonization of both epicontinental and epioceanic areas in the western Tethys.
Around the ETMEE, the last genera of this group (Salgirella and Cirpa) became extinct. They share a Mediterranean origin, but diversification of Salgirella occurred in epioceanic habitats in a twofold pathway (east- and westwards), even passing through bioprovince boundaries, whereas speciation of Cirpa also was completed in epicontinental habitats within the Euro-Boreal Bioprovince, until their extinction in the ETMEE, just prior to the onset of the severe warming occurred in the basal Serpentinum Zone. Ecological perturbations in the first stages of ETMEE led to the migration of wellerelloids towards the proto-Atlantic margin (just as occurred with other brachiopod clades, such as Athyridida, Norelloidea, or Spiriferinida), colonizing westernmost, probably colder habitats, to escape the hyperwarming event.
It has been substantiated that the rhynchonellide eurinoid/septifal/ribbed morpho-group was resilient around ecological crises in epicontinental areas. Thus, wellerelloids are the only large rhynchonellides in the extinction interval in many areas. The eurinoid/septifal/ribbed morpho-group represented by Cirpinae also is recorded in this area after the extinction boundary through the genus Pseudogibbirhynchia. A possible phyletic relationship has been argued between the last pre-ETMEE representative of Cirpinae (Cirpa) and post-ETMEE Pseudogibbirhynchia taxa.
A new species of rhynchonellide (Cirpa lucentina) is erected and the Cirpinae taxa around the ETMEE from the peri-Iberian paleomargins are taxonomically revised. The nominal species, Rhynchonella fallax (Deslongchamps), formerly assigned to different genera, is now confirmed as belonging to the genus Cirpa. Likewise, morpho-biometrical and internal structure analyses support the validity of taxonomic separation between the genera Cirpa and Salgirella, adding new supplementary diagnostic criteria for this purpose.
Acknowledgments
This research is a contribution to the IGCP-710 Western Tethys meets Eastern Tethys, and to the Research Groups VIGROB-167 (University of Alicante) and PBM-910431 (Complutense University of Madrid). Authors thank the staff of MUPE for access to the Peiró collection, where several specimens of Salgirella? goicoecheai are deposited. Critical reviews and helpful comments of the editors, M. Manceñido, and an anonymous reviewer clearly improved the quality of this paper.
Data availability statement
Data available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.vmcvdncvr.


















