Introduction
The Tanis site of North Dakota has been of much interest recently because it is thought to contain the horizon from ca. 66 Ma in which the Chicxulub asteroid had recently struck off the Yucatan coast causing one of this planet's great extinction events (DePalma et al., Reference DePalma, Smit, Burnham, Kuiper and Manning2019, Reference DePalma, Oleinik, Gurche, Burnham and Klingler2021). The impact of the asteroid marked the end of the Cretaceous and the beginning of the Paleocene, and it is thought by some paleontologists to have led to the extinction of 70% of the Earth's species (Jablonski, Reference Jablonski1994). The Tanis site is of particular interest to us because it contains a mass mortality of well-preserved fossil fishes, preserved as nearly complete, fully three-dimensional skeletons. The site where the fishes were collected contains large sturgeon and paddlefish skeletons of a meter or more in length. Preparation of these specimens from their sandstone matrix was a long and difficult process, requiring several years of effort by two museum preparators of the Field Museum. However, the result of that effort was four well-preserved sturgeons belonging to two species described here, as well as a number of specimens of a new paddlefish species that will be described in a later publication.
The sturgeon family Acipenseridae and the paddlefish family Polyodontidae (see Grande and Bemis, Reference Grande and Bemis1991; Grande et al., Reference Grande, Jin, Yabumoto and Bemis2002) are the only extant families of Acipenseriformes. The order also includes the extinct families †Peipiaosteidae (Late Jurassic to Early Cretaceous, Asia; see Hilton et al., Reference Hilton, Grande and Fan2021) and †Chondrosteidae (Jurassic, Europe; see Hilton and Forey, Reference Hilton and Forey2009) (Grande and Bemis, Reference Grande, Bemis, Stiassny, Parenti and Johnson1996; Bemis et al., Reference Bemis, Findeis and Grande1997; Hilton and Grande, Reference Hilton, Grande and Schultze2021). Acipenseriformes has been interpreted as the closest extant sister group of the Neopterygii (i.e., gars, bowfins, and teleosts; e.g., Patterson, Reference Patterson1982, Giles et al., Reference Giles, Xu, Near and Friedman2017, Hughes et al., Reference Hughes, Ortí, Huang, Sun and Baldwin2018; see also Grande and Bemis, Reference Grande and Bemis1998 and Grande, Reference Grande2010), with recent phylogenetic analyses reexamining this question (e.g., Argyriou et al., Reference Argyriou, Giles, Friedman, Romano, Kogan and Sánchez-Villagra2018, Reference Argyriou, Giles and Friedman2022). Regardless of their exact phylogenetic position at the base of Actinopterygii, this order remains the most taxonomically rich clade of extant actinopterygian fishes outside of Neopterygii. Therefore, there has been substantial interest in the evolutionary history of Acipenseriformes (Bemis et al., Reference Bemis, Findeis and Grande1997) and Acipenseridae in particular (Hilton et al., Reference Hilton, Grande and Bemis2011).
The bauplan of the extant Acipenseridae, including an elongate body, a weakly ossified axial skeleton, and a strongly heterocercal tail flanked dorsally by rhombic scales, resembles that of many fossil lineages of basal actinopterygians, thereby giving them the moniker of so-called “living fossils.” There are ~25 living species of Acipenseridae distributed in rivers, lakes, and near-shore coastal waters throughout the Northern Hemisphere, including North America, Europe, and Asia. Four widely recognized extant genera are classified within the family, although there are significant taxonomic and phylogenetic uncertainties within the family. The four extant acipenserid genera are Pseudoscaphirhynchus Nikolskii, Reference Nikolskii1900 (three species, Central Asia), Scaphirhynchus Heckel, Reference Heckel1836 (three species, North America), Huso Linnaeus, Reference Linnaeus1758 (two species, Europe and Asia), and Acipenser Linnaeus, Reference Linnaeus1758 (at least 17 species, North America, Asia). Both Huso and Acipenser are demonstrably non-monophyletic (see Hilton et al., Reference Hilton, Grande and Bemis2011), with the two species of Huso nested within Acipenser and sister to different groups within Acipenser (i.e., a paraphyletic Huso; Vasil'eva et al., Reference Vasil'eva, Vasil'ev, Shedko and Novomodny2009). Pseudoscaphirhynchus, although regarded as monophyletic, is also nested within Acipenser as sister to Acipenser stellatus Pallas, 1771 (Birstein et al., Reference Birstein, Doukakis and DeSalle2002; Hilton et al., Reference Hilton, Grande and Bemis2011, Reference Hilton, Dillman, Paraschiv and Suciu2022).
Although the relationships among sturgeon species are unresolved, and the most species-rich genus (Acipenser) is demonstrably not monophyletic, Acipenseridae is considered to be monophyletic based on both genetic (Birstein et al., Reference Birstein, Doukakis and DeSalle2002; Dillman et al., Reference Dillman, Wood, Kuhajda, Ray, Salnikov and Mayden2007, Krieger et al., Reference Krieger, Hett, Fuerst, Artyukhin and Ludwig2008) and morphological (Hilton et al., Reference Hilton, Grande and Bemis2011) data, and is defined by: (1) presence of a laterally curving rostral canal; (2) a single posterior ventral rostral bone; (3) branchiostegals of varying shapes; (4) dorsalmost branchiostegals pillar-like and laterally concave; (5) presence of a palatal complex; (6) presence of an anterior shelf on hypobranchial 1; (7) dorsal scutes between skull and the dorsal fin; (8) supracleithrum reaching anterior to the level of extrascapulars; (9) presence of a cardiac shield formed by the shoulder girdle; (10) a tight, interdigitating suture between the clavicle and cleithrum; and (11) presence of a supracleithral cartilage (Hilton et al., Reference Hilton, Grande and Bemis2011). Several other characteristics are identified in support of monophyly of the family, although these are homoplastically present in other Acipenseriformes. These include the presence of an “acipenserid” type of pectoral spine (a pectoral spine is also found in †Protopsephurus, Grande et al., Reference Grande, Jin, Yabumoto and Bemis2002; †Chondrosteus, Hilton and Forey, Reference Hilton and Forey2009; and †Yanosteus, Jin et al., Reference Jin, Tian, Yang and Deng1995; Jin, Reference Jin, Chen and Jin1999; Hilton et al., Reference Hilton, Grande and Fan2021), a cleithral process that limits the mobility of pectoral fin spine (Findeis, Reference Findeis1997), and having a series of thick, diamond-shaped scutes along the lateral line (these are not found in †Priscosturion; Grande and Hilton, Reference Grande and Hilton2006).
The fossil record of Acipenseridae extends from at least the Late Cretaceous (Hilton and Grande, Reference Hilton and Grande2006), but a ghost lineage extends from at least the Early Cretaceous from when the earliest Polyodontidae are known (Hilton et al., Reference Hilton, Grande and Bemis2011). Most fossil acipenserid remains are highly fragmentary and cannot be diagnosed differentially to genus or species levels (Hilton and Grande, Reference Hilton and Grande2006). Articulated fossil sturgeons are relatively rare, and include the monotypic genera †Protoscaphirhynchus Wilimovsky, Reference Wilimovsky1956 (Late Cretaceous, Hell Creek Formation, Montana), †Priscosturion Grande and Hilton, Reference Grande and Hilton2009 (Late Cretaceous, Judith River Formation, Montana), †Engdahlichthys Murray et al., Reference Murray, Brinkman, Demar and Wilson2020 (Lower Paleocene, Tullock Member of Fort Union Formation, Montana), and †Anchiacipenser Sato et al., Reference Sato, Murray, Vernygora and Currie2018 (Late Cretaceous, Dinosaur Park Formation, Alberta. The purpose of this paper is to review the fossil record of Acipenseridae from the Late Cretaceous of North America, and to describe two new species from the Hell Creek Formation (Fig. 1).
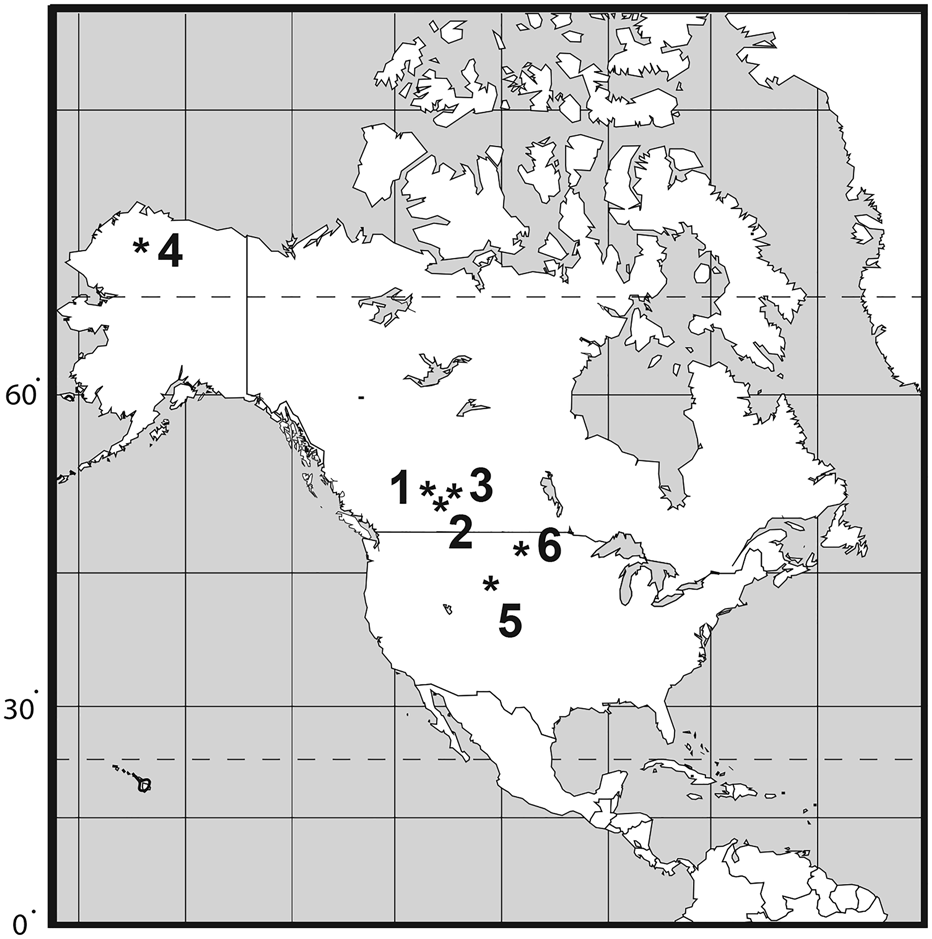
Figure 1. Map indicating distribution of Late Cretaceous Acipenseridae in North America. (1) Milk River Formation (Santonian–Campanian); (2) Judith River Group (middle–late Campanian); (3) Dinosaur Park Formation, late Campanian; (4) Prince Creek Formation (early Maastrichtian); (5) Lance Formation (late Maastrichtian); (6) Hell Creek Formation (late Maastrichtian).
Materials and methods
Preparation, photography, and illustration
The specimens described herein were mechanically prepared under stereo microscopes using pin vices with sharp-point needles. They were then photographed using a Canon EOS 5D digital camera. Some specimens were coated with ammonium chloride sublimate prior to photography to enhance contrast. Line drawings were rendered by hand by EJH based on these images.
Counts, measurements, and style
Counts and measurements follow Hilton et al. (Reference Hilton, Grande and Bemis2011). In cases in which a measurement could not be made due to incomplete preservation, the measurement was recorded as estimated (est.). Daggers (†) precede the names of taxa known exclusively as fossils.
Repositories and institutional abbreviations
Materials that serve as the basis for description of the new species are referred to in the relevant sections below. Comparative materials are listed in Grande and Bemis (Reference Grande and Bemis1991, Reference Grande, Bemis, Stiassny, Parenti and Johnson1996), Grande et al. (Reference Grande, Jin, Yabumoto and Bemis2002), Hilton and Forey (Reference Hilton and Forey2009), and Hilton et al. (Reference Hilton, Grande and Bemis2011, Reference Hilton, Dillman, Paraschiv and Suciu2022).
AMNH, American Museum of Natural History, New York, NY, U.S.A.; CMN, Canada Museum of Nature, Ottawa. Canada; DMNH EVP, Denver Museum of Nature and Science, Denver, CO, U.S.A.; FMNH, Field Museum, Chicago, IL U.S.A.; MOR, Museum of the Rockies, Montana State University, Bozeman, MT, U.S.A.;UALVP, University of Alberta Laboratory of Vertebrate Paleontology, Alberta, Canada; UMMP, University of Michigan Museum of Paleontology, Ann Arbor, MI, U.S.A.; UWBM, University of Washington Burke Museum, Seattle, WA, U.S.A.
Systematic paleontology
Class Osteichthyes Huxley, Reference Huxley1880
Subclass Actinopterygii Cope, Reference Cope1887
Infraclass Chondrostei Müller, Reference Müller1844
Order Acipenseriformes Berg, Reference Berg1940
Family Acipenseridae Bonaparte, Reference Bonaparte1831
Genus Acipenser Linnaeus, Reference Linnaeus1758
Type species
Acipenser sturio Linnaeus, Reference Linnaeus1758.
†Acipenser praeparatorum new species
Figures 2–17
Holotype
FMNH PF17608 (Fig. 2.1). Body fossil specimen with skull, pectoral girdle and fin, and dorsal, lateral, and ventral scute rows of the anterior portion of the body exposed in lateral view. Dorsal, pelvic, anal, and caudal fins missing. Preserved portion of the individual measures 89 cm. Total length is estimated to have been ~120 cm.
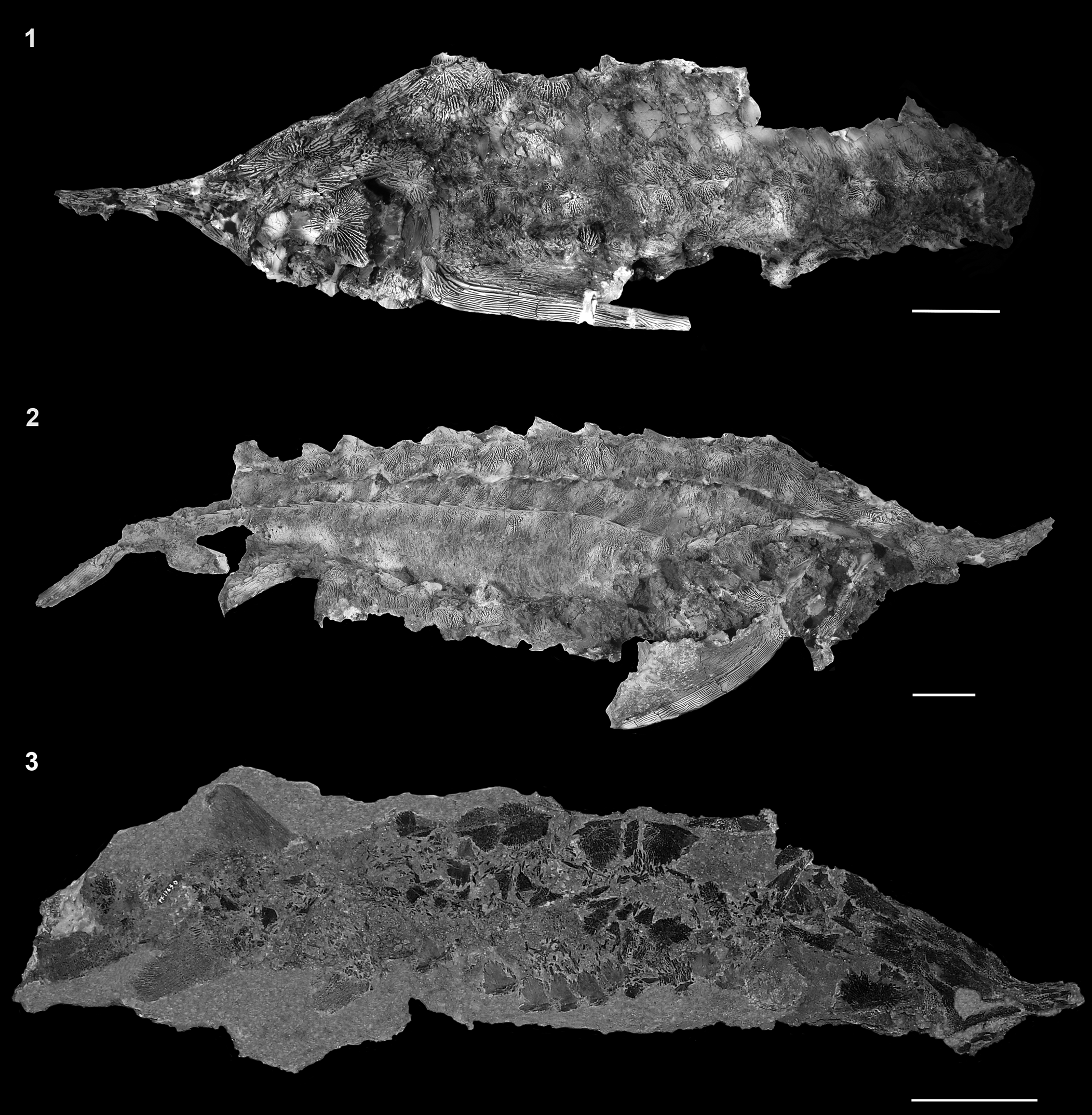
Figure 2. Full body specimens of †Acipenser praeparatorum n. sp. (1) FMNH PF17608 (holotype); (2) FNMH PF17607; (3) FMNH PF17630. Anterior facing left in (1) and anterior facing right in (2, 3). Scale bars = 5 cm.
Paratype specimens examined
FMNH PF17630, articulated skeleton exposed in right lateral view (missing posterior portion of the caudal fin); FMNH PF17609, isolated skull with both dorsal and ventral surfaces exposed. DMNH EVP.138511, articulated skeleton exposed in left lateral view (skull and portions of the body obscured by three overlying polyodontid rostra [DMNH EVP.138509, DMNH EVP.138510, DMNH EVP.138512]; caudal fin missing posteriorly).
In addition to these body specimens, several specimens representing isolated or partial collections of bones were examined and illustrated.
Diagnosis
An acipenserid that differs from all other known species in having an extremely narrow and elongate dorsal branchiostegal that lacks superficial ornamentation. The dorsalmost branchiostegal of other species of Acipenser, Huso, and Pseudoscaphirhynchus is relatively elongate and tall (e.g., Acipenser brevirostrum Lesueur, Reference Lesueur1818; Hilton et al., Reference Hilton, Grande and Bemis2011, figs. 47–51), in contrast to the triangular condition found in Scaphirhynchus. The height-to-width ratio of the branchiostegal in †Acipenser praeparatorum n. sp. is much greater than in other acipenserid taxa (e.g., ~3.0 vs. 2.4 as shown in A. brevirostrum in Hilton et al., Reference Hilton, Grande and Bemis2011, fig. 48). Other acipenserids also have ornamentation on the superficial surface of the ventral end of the dorsalmost branchiostegal.
Occurrence
Tanis site (DePalma et al., Reference DePalma, Smit, Burnham, Kuiper and Manning2019) of the Hell Creek Formation, Bowman County, North Dakota, U.S.A.; Late Cretaceous (Maastrichtian). This site includes the transition point between Cretaceous and Paleocene time and is thought by some authors to have fossilized traces of the Chicxulub impact (as mentioned in the Introduction). Some authors claim that the gills of the sturgeon fossils from the site have ejecta spherules from the impact (DePalma et al., Reference DePalma, Smit, Burnham, Kuiper and Manning2019), although we were unable to verify this with our material. Rocks containing the type and figured material used in our study were collected by Steve Nicklas and Robert Coleman in 2010.
Description
Snout pointed, ~42–49% of the head length, with a subterminal, edentulous mouth; five rows of dermal scutes; pectoral fin large with robust spine along it; ornamentation of dermal bones formed by well-defined ridges that radiate from a presumed center of ossification, with ridges forming deep channels; snout covered dorsally by elongate dorsal rostral bones and laterally by border rostral bones; jugal with elongate horizontal arm and a very slight median shelf; ornamentation of the dermosphenotic is more reticulate and has less-prominent ridges than that of most other skull roofing bones; skull roof posterior to the snout is formed by the paired nasals, antorbitals, frontals, parietals, dermosphenotics, dermopterotics, and the median extrascapular, and a variable number of lateral extrascapulars; antorbital curves slightly ventrally, but does not have an unornamented descending process; left and right frontals are separated from one another by the posterior dorsal rostral bones; median extrascapular more oval than in extant acipenserids; first dorsal scute is massive compared to the more-posterior dorsal scutes; subopercle is rounded posteriorly and has a distinct anterior extension; a single branchiostegal is exceptionally tall and slender; dermopalatine has a distinct median blade or shelf; medial tip of the dentary is broader than the lateral tip and has a well-developed shelf; ~38 dorsal-fin rays; caudal fin with series of dorsal caudal fulcra (number unknown), anteriormost is almond-shaped with deep ridges on its dorsal surface; more posterior dorsal caudal fulcra are deeply forked; pectoral-fin spine thick and robust (57–83% head length); body armored by five rows of scutes as well as bony scales intercalated between the rows of scutes; all scutes are heavily ornamented and bear a prominent, sharply pointed ridge, often developed into a well-developed thorn-shaped spine; 11 dorsal scutes; 41 lateral scutes; lateral scutes become progressively smaller from anterior to posterior, but all are tall (depth-to-width ratio ~39–45%); ornamentation of the lateral scutes formed anteriorly by a series of ridges of bone radiating from the central ridge or spine and posteriorly by horizontally oriented ridges of bone; body scales between the scutes tall, overlapping, and irregularly shaped, with horizontally arranged rows of small, tooth like ornamentation.
Anatomical description
In this section, we provide a detailed description of †Acipenser praeparatorum n. sp. organized by region of the body.
General body form and dermal bone ornamentation.—The general body form of †Acipenser praeparatorum n. sp. is similar to that of all other Acipenseridae, with the exception of †Priscosturion longipinnis (Grande and Hilton, Reference Grande and Hilton2006). The snout is elongate and pointed, with a subterminal, edentulous mouth. In the three specimens for which the head length and preorbital length can be estimated with confidence, the preorbital (i.e., snout) length is ~42–49% of the head length. There are five rows of dermal scutes, including a median dorsal series, paired lateral series, and paired ventral series. The pectoral fin is large, and bears a long, robust spine along its leading edge. Although four moderately complete specimens were examined, the posterior portion of the bodies were poorly preserved, so that body proportions and most of the fin positions are unknown.
The ornamentation of the dermal bones, including those forming the skull roof, pectoral girdle, and scutes, is formed by well-defined ridges that radiate from a presumed center of ossification (Fig. 3). Some of the ridges anastomose, but they do not form networks of cells, as many other acipenserids do (e.g., Acipenser oxyrinchus Mitchill, Reference Mitchill1815). The ridges also form deep channels, as seen in some other members of the family (EJH, personal observation). Element-specific variations in the ornamentation are described below.
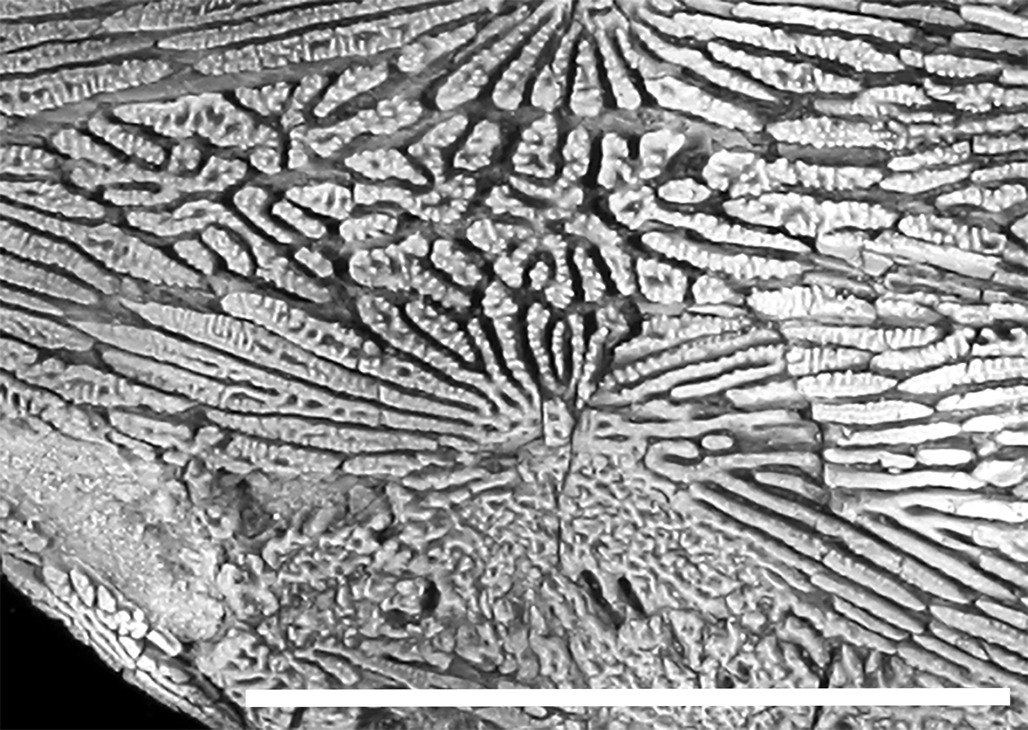
Figure 3. Close up of skull roof of †Acipenser praeparatorum n. sp. (FMNH PF17609) paratype showing detail of the ornamentation of the dermal bones. Scale bar = 5 cm.
Dermal bones of the skull
The snout of †Acipenser praeparatorum n. sp. is covered dorsally by elongate dorsal rostral bones and laterally by a series of border rostral bones (Figs. 4, 5), which are separated by openings that presumably housed rosettes of ampullary organs, as in extant sturgeons (Fig. 6; Hilton et al., Reference Hilton, Grande and Bemis2011). The posteriormost border rostral bone contacts the jugal. The jugal has an elongate horizontal arm, with a short vertical arm that contacts the postorbital (Fig. 4). The median surface of the jugal bears a very slight median shelf (Figs. 7–9) that contacts the series of rostral canal bones; this series has a similar course that arches laterally around the presumed position of the barbles before running parallel to the ventral rostral bones to the tip of the snout (Fig. 7). The post orbital is flared dorsally to contact the dermosphenotic anteriorly and the dermopterotic posteriorly (Figs. 4, 6). The dermosphenotic is large, and broadly contacts the frontal medially and the antorbital anteriorly. The ornamentation of the dermosphenotic is more reticulate and has less-prominent ridges than that of most other skull roofing bones (the posttemporal has a similar ornamentation on its anterolateral portion).

Figure 4. †Acipenser praeparatorum n. sp. (FMNH PF17608, holotype), skull in lateral view. (1) Photograph and (2) line drawing. Anterior facing left. Abbreviations: ao, antorbital; br, branchiostegal; brb, border rostral bones; cl, cleithrum; clv, clavicle; d, dentary; dpl, dermopalatine; dpt, dermopterotic; drb, dorsal rostral bone; ds1, first dorsal scute; dsp, dermosphenotic; excl, lateral extrascapular; excm, median extrascapular; fr, frontal; ih, interhyal; j, jugal; n, nasal; pa, parietal; pcl, postcleithrum; pfs, pectoral-fin spine; po, postorbital; ppt, palatopterygoid; pt, posttemporal; scl, supracleithrum; sop, subopercle; vrb, ventral rostral bone. Scale bars = 5 cm.
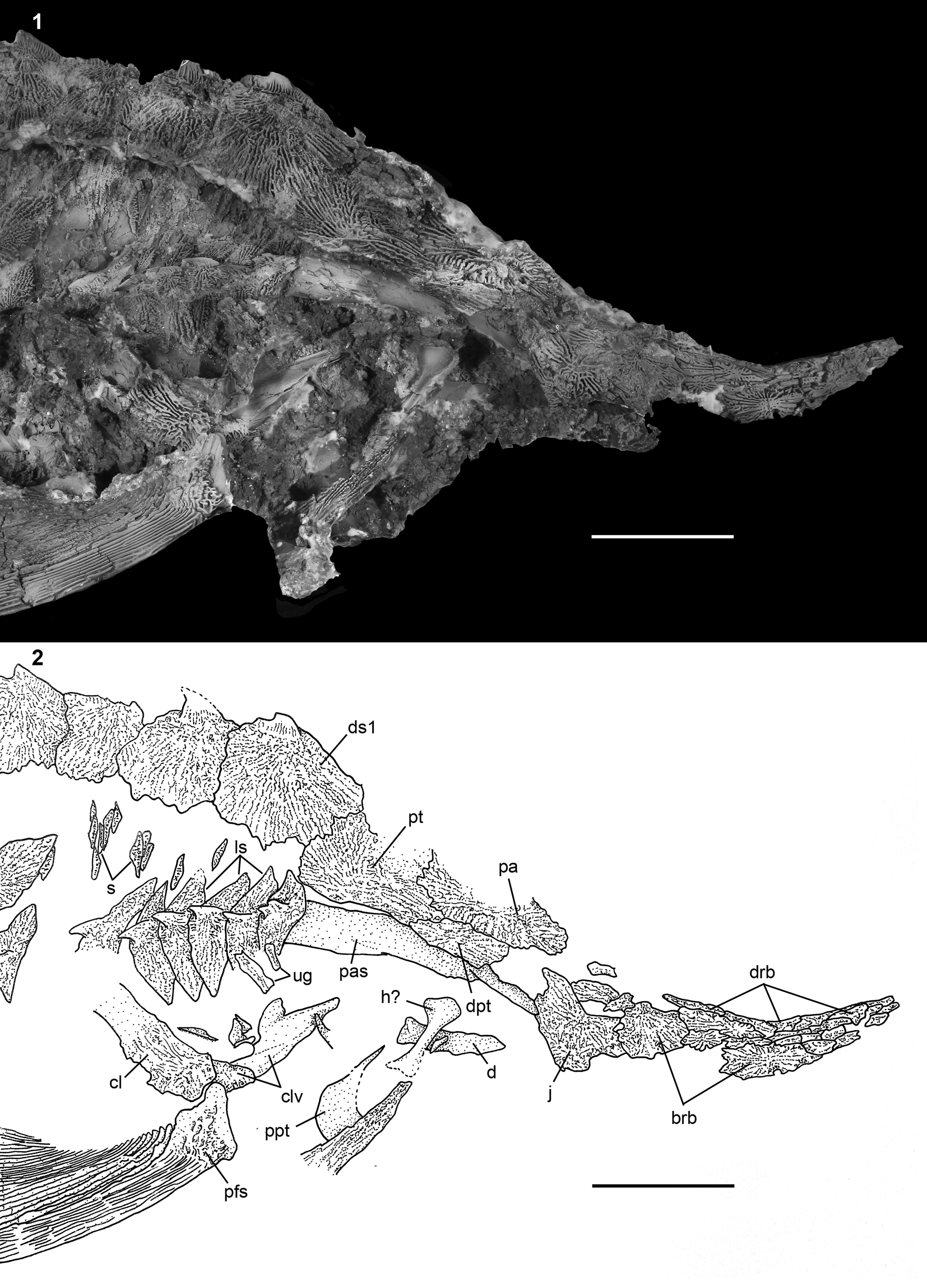
Figure 5. †Acipenser praeparatorum n. sp. (FMNH PF17607), skull in lateral view. (1) Photograph and (2) line drawing. Anterior facing right. Abbreviations: brb, border rostral bones; cl, cleithrum; clv, clavicle; d, dentary; dpt, dermopterotic; drb, dorsal rostral bone; ds1, first dorsal scute; h, hyomandibula; j, jugal; ls, lateral scute; pa, parietal; pas, parasphenoid; pfs, pectoral-fin spine; ppt, palatopterygoid; pt, posttemporal; s, body scales (dermal bony plates between scutes); ug, unidentified gill arch bone. Scale bars = 5 cm.
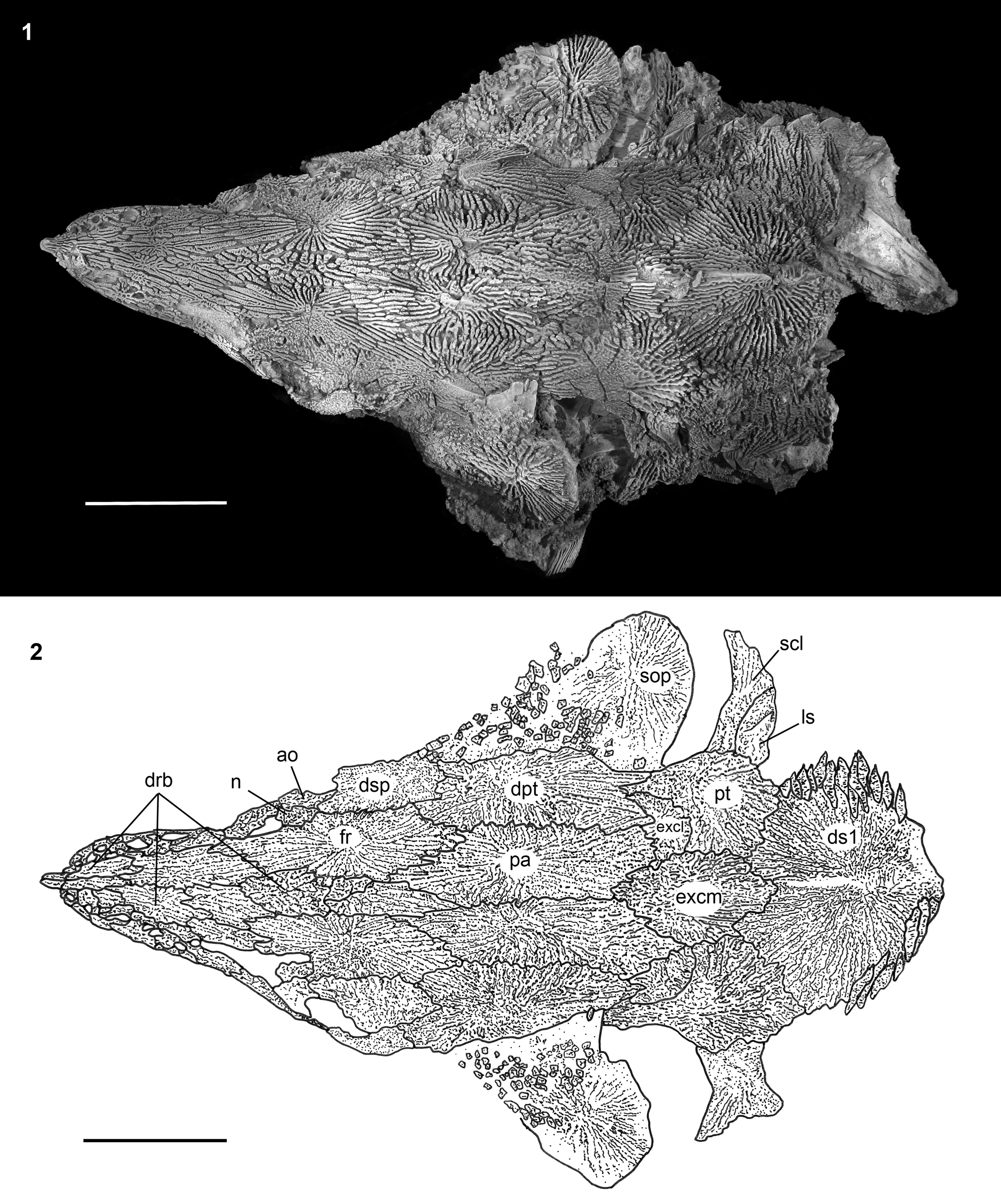
Figure 6. †Acipenser praeparatorum n. sp. (FMNH PF17609), paratype skull in dorsal view. (1) Photograph and (2) line drawing. Anterior facing left. Abbreviations: ao, antorbital; dpt, dermopterotic; drb, dorsal rostral bone; ds1, first dorsal scute; dsp, dermosphenotic; excl, lateral extrascapular; excm, median extrascapular; fr, frontal; ls, lateral scute; n, nasal; pa, parietal; pt, posttemporal; scl, supracleithrum; sop, subopercle. Scale bars = 5 cm.
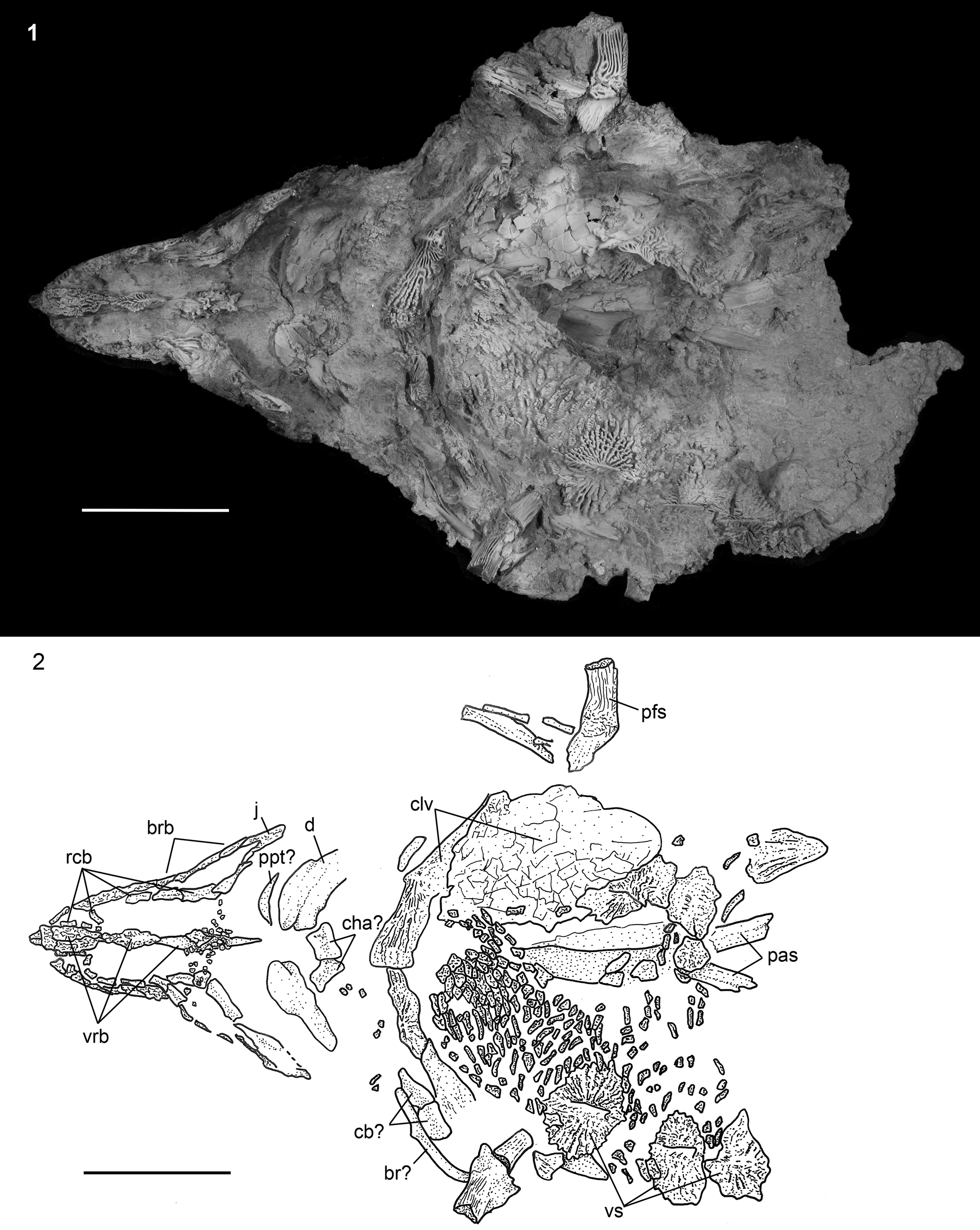
Figure 7. †Acipenser praeparatorum n. sp. (FMNH PF17609), paratype skull in ventral view. (1) Photograph and (2) line drawing. Anterior facing left. Abbreviations: br, branchiostegal; brb, border rostral bones; cb, certatobranchial; cha, anterior ceratohyal; clv, clavicle; d, dentary; j, jugal; pas, parasphenoid; pfs, pectoral-fin spine; ppt, palatopterygoid; rcb, rostral canal bones; vrb, ventral rostral bone; vs, ventral scute. Scale bars = 5 cm.
The skull roof posterior to the snout is formed by the paired nasals, antorbitals, frontals, parietals, dermosphenotics, dermopterotics, the median extrascapular, and a variable number of lateral extrascapulars. As typical for most acipenserids, the posttemporals and the first dorsal scute are tightly incorporated into the skull roof (Figs. 4–6). The nasals, preserved in FMNH PF17609 (Fig. 6) and possibly FMNH PF17608 (Fig. 4), are rectangular and flat and contact the medial edge of the antorbital; elements corresponding to the tubular nasals (Hilton et al., Reference Hilton, Grande and Bemis2011) are not preserved. The antorbital is positioned at the anterodorsal corner of the orbit and curves slightly ventrally, although it does not have an unornamented descending process as found in most Acipenseridae. The frontals are somewhat rhombic in shape (Fig. 6). The left and right frontals are separated from one another by the posterior dorsal rostral bones.
The dermopterotics (laterally) and parietals (medially) form most of the skull roof posterior to the orbits (Fig. 6). The dermopterotic forms the lateral margin of the skull roof and carries the supratemporal sensory canal. Part of its dorsal surface is unornamented anteriorly and posteriorly (Figs. 8, 9) where it was overlapped by more superficial bones. The left and right parietals contact one another along the midline of the skull roof (Fig. 6). There are unornamented regions both anteriorly and posteriorly on the dorsal surface of the parietals (Figs. 8, 9) that correspond to surfaces that are overlain by other skull roofing bones, as seen in extant sturgeons (Hilton et al., Reference Hilton, Grande and Bemis2011, fig. 12). Posterior to the parietals, the median extrascapular is positioned along the midline of the skull roof (Fig. 6). This element is best preserved in FMNH PF17609, and more oval in outline that that found in extant acipenserids, in which it is more triangular in shape (e.g., Hilton et al., Reference Hilton, Grande and Bemis2011, fig. 12). In FMNH PF17609 (Fig. 6), there are two lateral extrascapulars, one on either side of the median extrascapular; only FMNH PF17608 (Fig. 4) has an element preserved that is tentatively identified as a lateral scapular. The ornamentation on the lateral extrascapulars is more condensed and not ridge-like in comparison to other dermal bones of the skull roof. In FMNH PF17607, based on the ornamentation patterns, it appears that the lateral extrascapular carries the confluence of the trunk, occipital, and supratemporal sensory canals, although this needs to be confirmed with additional specimens.
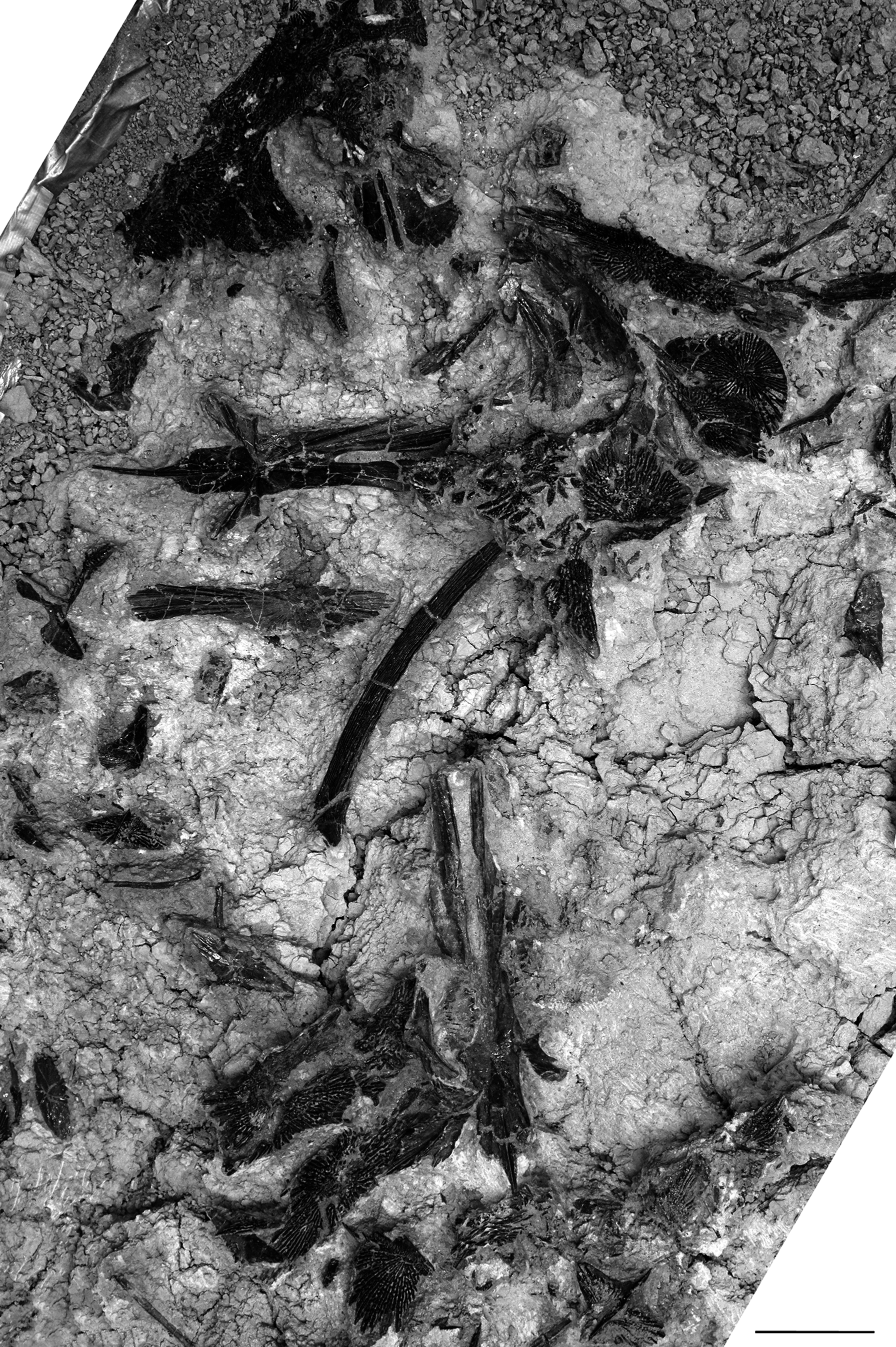
Figure 8. Disarticulated bones of several specimens of †Acipenser praeparatorum n. sp. preserved together and photographed in situ (including specimens cataloged as FMNH PF17622, PF17623, PF17624, PF17625, and PF17614). Scale bar = 5 cm.
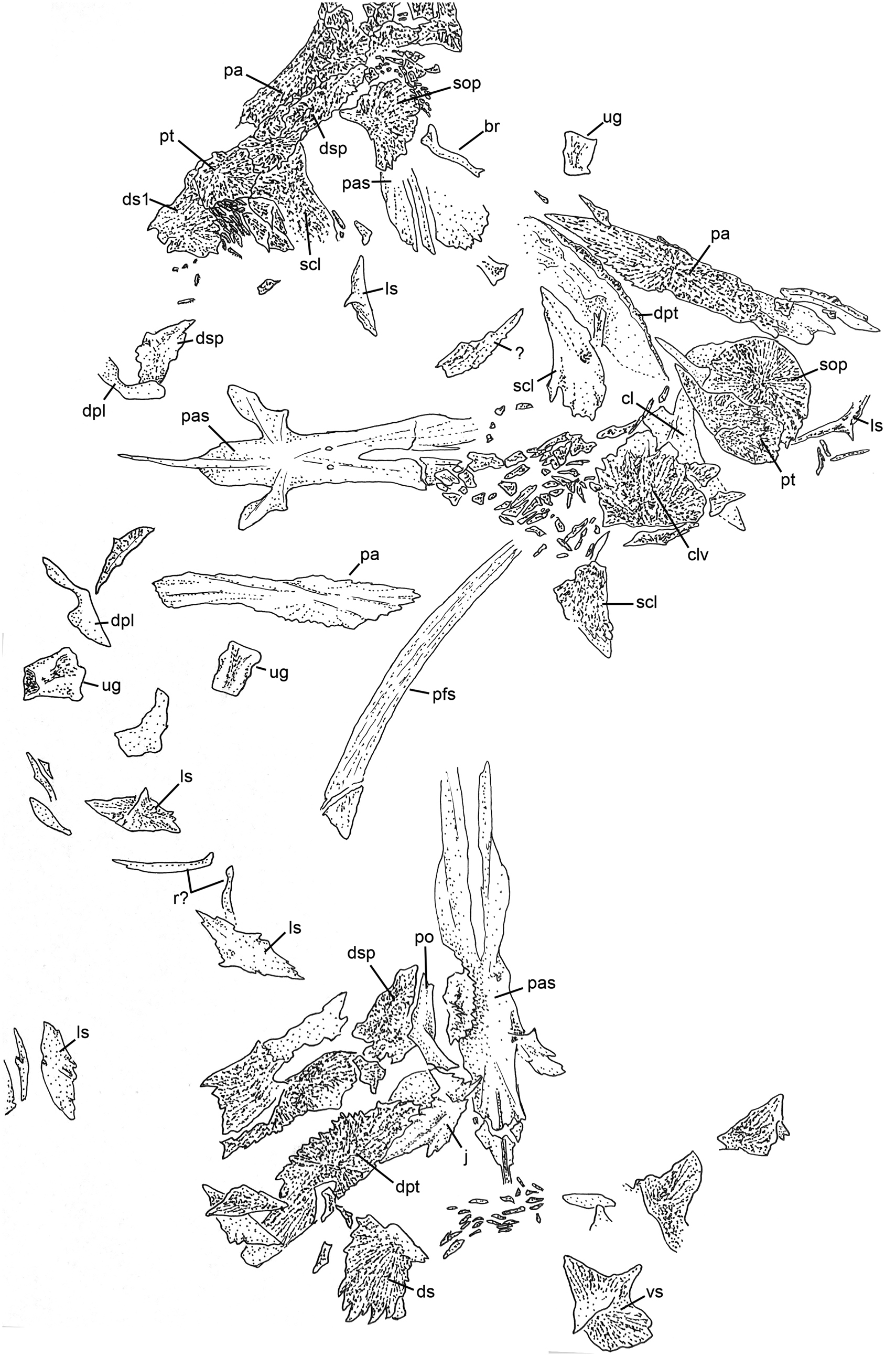
Figure 9. Line drawing of Figure 3. Abbreviations: br, branchiostegal; cl, cleithrum; clv, clavicle; dpl, dermopalatine; dpt, dermopterotic; ds, dorsal scute; ds1, first dorsal scute; dsp, dermosphenotic; j, jugal; ls, lateral scute; pa, parietal; pas, parasphenoid; pfs, pectoral-fin spine; po, postorbital; pt, posttemporal; r, rib; scl, supracleithrum; sop, subopercle; ug, unidentified gill arch bone; vs, ventral scute. Scale bar = 5 cm.
The posttemporal and the first dorsal scute are described in this section because they are both incorporated into the skull roof. The posttemporal forms the posterolateral corner of the skull roof. It bears a distinct anterolateral extension that contacts the dermopterotic. Posteriorly, it is rounded and broadly contacts the anterior margin of the first dorsal scute. The posttemporal bears a slight ridge just dorsal to its suture with the supracleithrum. The first dorsal scute is massive compared to the more posterior dorsal scutes. It bears an unornamented thorn-shaped spine similar to the other scutes, although it appears to be relatively shorter; this spine is best preserved in FMNH PF17607 (Fig. 5) and FMNH PF17609 (Fig. 6).
The shape of the parasphenoid of †Acipenser praeparatorum n. sp. is typical for the family Acipenseridae (Hilton et al., Reference Hilton, Grande and Bemis2011, figs. 43, 44), and is best seen as disarticulated specimens exposed in dorsal (FMNH PF17622) and ventral (FMNH PF17623) views (Fig. 10). The posterior arms of the parasphenoid, which define an aortic notch, extend far posteriorly. It is unclear how far they extended relative to the skull roof. The ascending processes of the parasphenoid project laterally from the body of the parasphenoid, and then are directed anteriorly along most of the length of the processes. There is a well-developed median anterior process of the parasphenoid (Fig. 10). In extant acipenserids, the median anterior process contacts the posteriormost ventral rostral bone (Hilton et al., Reference Hilton, Grande and Bemis2011, figs. 28, 39). Although the relationship between these bones is unclear for †Acipenser praeparatorum n. sp. due to preservation, it is likely that a similar relationship existed. The ventral rostral bones of †Acipenser praeparatorum n. sp. are formed by a series of elongate elements along the ventral midline of the snout. The posteriormost element is median and the longest of the series (Fig. 7). The anteriormost ventral rostral bones are paired.
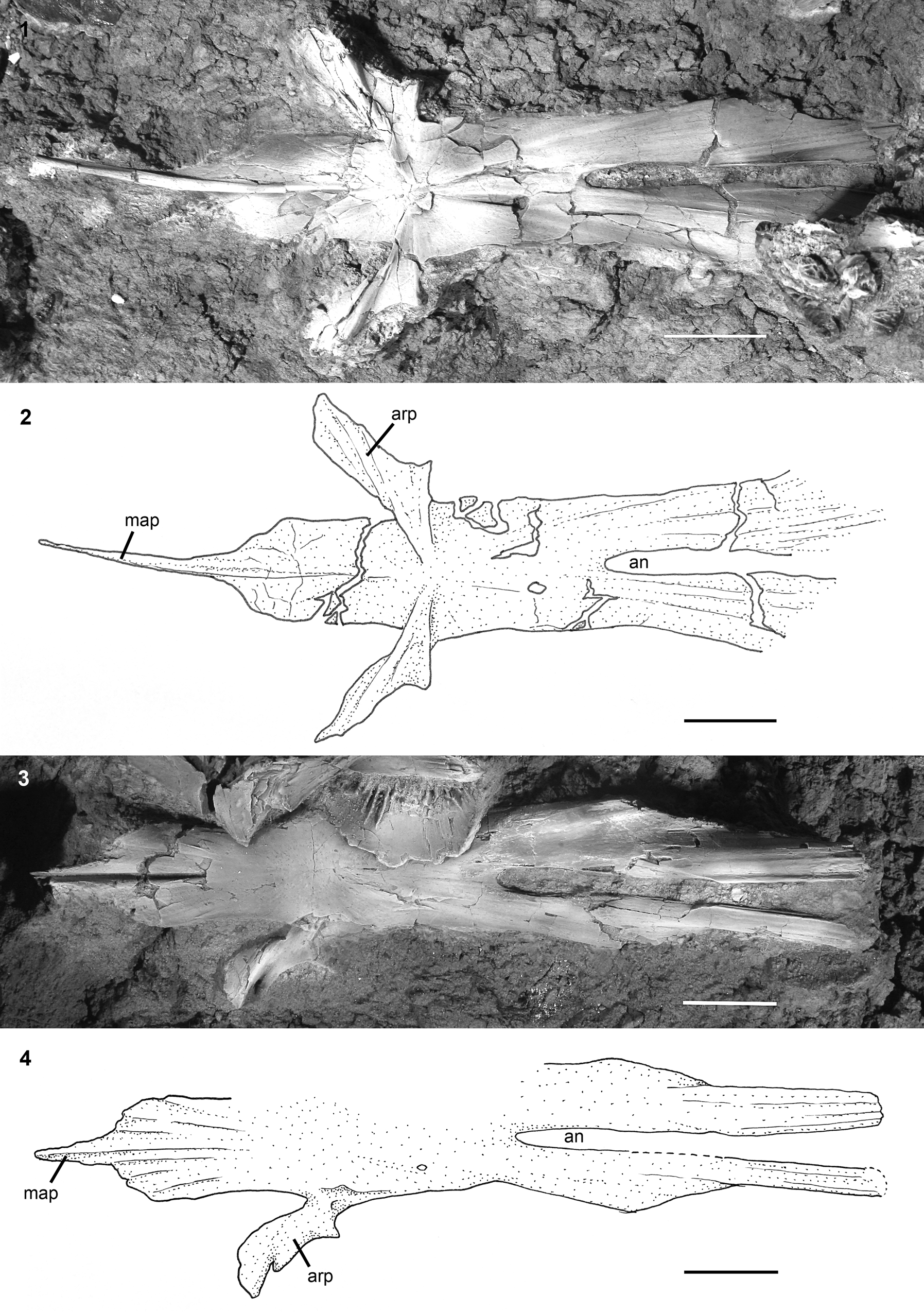
Figure 10. †Acipenser praeparatorum n. sp. isolated parasphenoid. (1) Photograph and (2) line drawing of parasphenoid in dorsal view (FMNH PF17622); (3) photograph and (4) line drawing of parasphenoid in ventral view (FMNH PF17623). Anterior facing left. Abbreviations: an, aortic notch; arp, ascending process of the parasphenoid; map, median anterior process of the parasphenoid. Scale bars = 2 cm.
Opercular series
The only elements of the opercular series preserved in †Acipenser praeparatorum n. sp. are the subopercle and a single branchiostegal. These elements are most clearly preserved in the holotype (Fig. 4), although the subopercle is also preserved as disarticulated specimens (Figs. 8, 9) and both the left and right subopercles are preserved in situ in FMNH PF17609 (Fig. 6); a branchiostegal is tentatively identified in this specimen (Fig. 7), as well as in a disarticulated specimen (Figs. 8, 9). The subopercle is rounded posteriorly, and has a distinct anterior extension (Figs. 8, 9). The anterior extension is broad and largely unornamented, as found in extant species of Acipenser (e.g., Hilton et al., Reference Hilton, Grande and Bemis2011, fig. 47). This region is covered by small bony plates (Fig. 6), as found in the cheek region of extant acipenserids (Hilton et al., Reference Hilton, Grande and Bemis2011, fig. 22).
Most species of Acipenseridae have at least two branchiostegals; a single concave element that contacts the medial surface of the subopercle, and at least one ventral element that is typically broader and ornamented (Hilton et al., Reference Hilton, Grande and Bemis2011). Only a single branchiostegal is preserved in †Acipenser praeparatorum n. sp., which corresponds to the more dorsal element of other sturgeons. It is likely that the absence of other branchiostegals is due to the lack of preservation. The branchiostegal of †Acipenser praeparatorum n. sp. is exceptionally tall and slender, and lacks superficial ornamentation (Fig. 4). It is slightly curved and articulates with the median surface of the subopercle through a shallow concavity on its lateral surface.
Jaws, suspensorium, and hyoid arch
The upper jaw of Acipenseridae comprises a dermopalatine along its anterior margin, a large palatopterygoid and autopalatine forming the roof of the mouth, an ectopterygoid, a quadratojugal, and a cartilaginous palatal complex (Hilton et al., Reference Hilton, Grande and Bemis2011, figs. 52, 53). The only elements preserved in our specimens of †Acipenser praeparatorum n. sp. are the dermopalatine and the palatopterygoid (for consistency with our other recent descriptions of the osteology of Acipenseridae [Grande and Hilton, Reference Grande and Hilton2006; Hilton et al., Reference Hilton, Grande and Bemis2011; Warth et al., Reference Warth, Konstantinidis, Naumann, Olsson and Hilton2017], we use dermopalatine here with the acknowledgment this element may represent the maxilla of other actinopterygians [Datovo and Rizzato, Reference Datovo and Rizzato2018]). The dermopalatine of †Acipenser praeparatorum n. sp. (Figs. 4, 8, 9, 11.3, 11.4) bears a distinct median blade or shelf that is exaggerated as found in Acipenser baerii Brandt, Reference Brandt1869, and A. transmontanus Richardson, Reference Richardson and Richardson1836 (Hilton et al., Reference Hilton, Grande and Bemis2011). The palatopterygoid of †Acipenser praeparatorum n. sp. is well developed and broad, with an elongate lateral process and a shorter but still distinct median process (Fig. 11.1, 11.2).
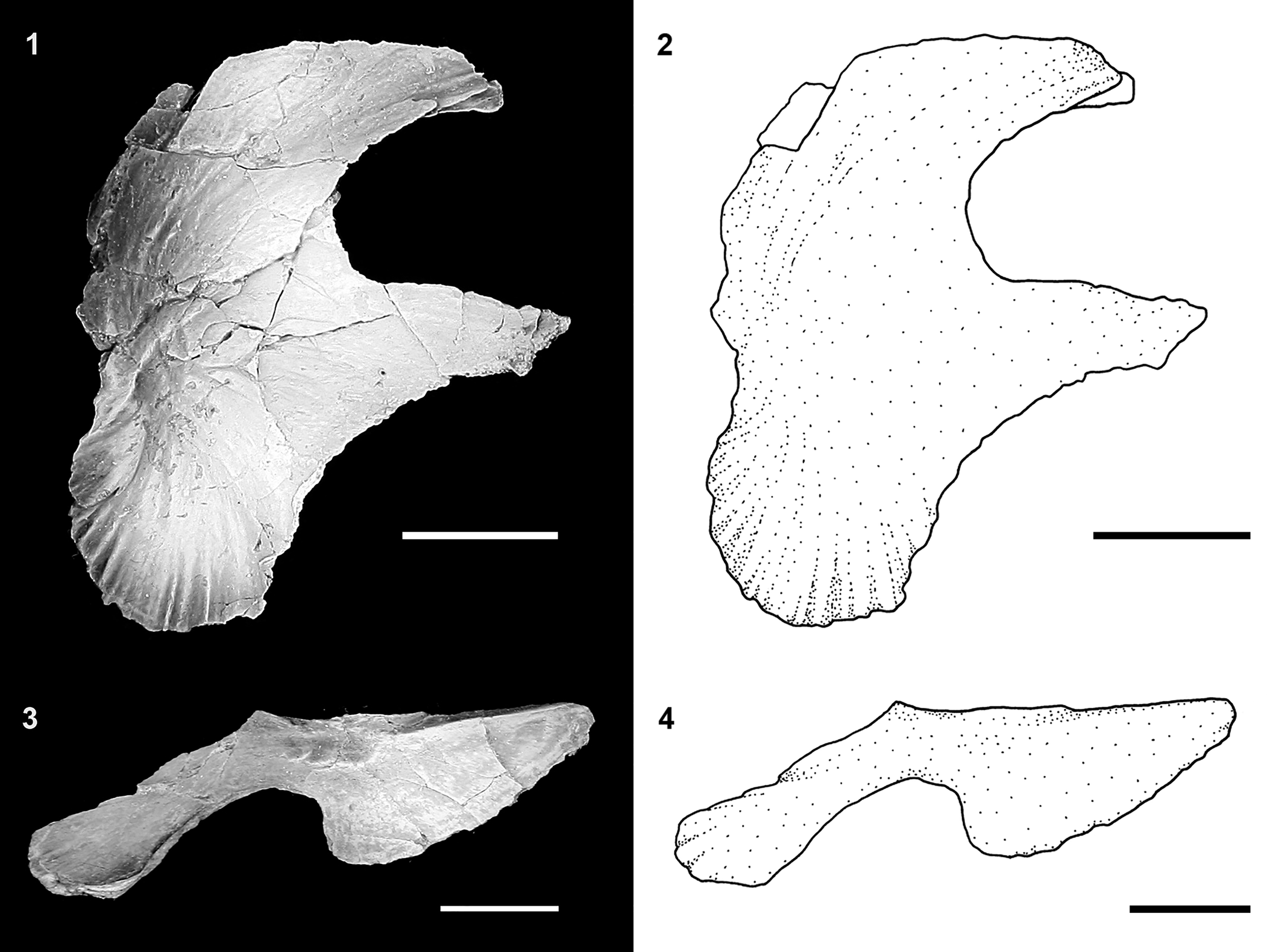
Figure 11. †Acipenser praeparatorum n. sp., isolated upper jaw bones. (1) Photograph and (2) line drawing of palatopterygoid in dorsal view (FMNH PF17610); (3) photograph and (4) line drawing of dermopalatine (maxilla) in lateral view (FMNH PF17614). Anterior facing right. Scale bars = 1 cm.
The only element of the lower jaw that is preserved in our specimens of †Acipenser praeparatorum n. sp. is the dentary (Figs. 4, 5, 7). This robust, thick bone is roughly rectangular in shape, but has a ventromedial process that gives its ventral margin a slightly concave outline (Fig. 4). The medial tip of the dentary is broader than the lateral tip, and the dentary has a well-developed shelf (Fig. 7), as found in other acipenserids (e.g., A. baerii; EJH, personal observation).
Very few elements of the hyoid arch are preserved. Surprisingly, the hyomandibula, typically the largest and most robust element of the hyoid arch, is represented by a single, tentatively identified element that provides few details of its morphology (Fig. 5). Other elements present in our specimens of †Acipenser praeparatorum n. sp. include a possible pair of anterior ceratohyals (Fig, 7) and a robust chondral bone that we interpret as the interhyal (Fig. 4). This bone is almost square in outline, but has an anteriorly directed process on its proximal end.
Gill arches
A few elements identified as gill arch bones are preserved (e.g., Figs. 5, 8, 9), but cannot be identified to series; two elements based on shape and position may represent ceratobranchials (Fig. 7). No gill rakers were found.
Vertebral column
No elements of the vertebral column of †Acipenser praeparatorum n. sp. are preserved, except for a few isolated elements that may represent ribs (Figs. 8, 9) and fragments of nine slender ribs in the mid-abdominal region (DMNH EPV.138511).
Dorsal and anal fins and supports
The dorsal and anal fins of †Acipenser praeparatorum n. sp. are poorly preserved in the available specimens. The dorsal and anal fins are best preserved in FMNH PF17630 (Fig. 2.3), which have ~38 and ~16 fin rays, respectively; DMNH EPV.138511 has ~38 dorsal-fin rays preserved, of which all are segmented and at least 13 appear to be branched. There are also ~23 proximal dorsal-fin radials that are exceptionally slender. A dorsal-fin fulcrum, which is preserved on DMNH EPV.138511, is ovoid and tapers to a point anteriorly.
Caudal fin and supports
Portions of the caudal fin and associated elements are preserved only in DMNH EPV.138511 (Fig. 12) and FMNH PF17630. In DMNH EPV.138511, there are eight dorsal caudal fulcra preserved from the anterior portion of the series (Fig. 12). The fulcra are similar in form to those of other acipenserids (Hilton et al., Reference Hilton, Grande and Bemis2011, fig. 83). The anteriormost dorsal caudal fulcrum is almond-shaped with deep ridges on its dorsal surface. Anterior to this fulcrum is a patch of broken ornamented dermal bone that appears to be a dorsal caudal peduncle scute or series of scutes. Disarticulated fulcra show the distinct, deeply notched form (Fig. 13.1) similar to posterior dorsal caudal fulcra of other Acipenseridae (e.g., Hilton, Reference Hilton, Arratia, Wilson and Cloutier2004, fig. 5B). Other isolated fulcra taper both anteriorly and posteriorly, and likely represent the anteriormost dorsal caudal fulcrum or the ventral caudal fulcrum (Fig. 13.2), as found in other members of the family. FMNH PF17608 also preserves the ventral margin of the caudal fin, including the single ventral caudal fulcrum and the bases of 13 unbranched fin rays. The flank of the dorsal lobe of the caudal fin supported a series of rhombic caudal scales (Fig. 12), although in no specimen are there more than a few scales preserved. The bases of seven hypurals are preserved on DMNH EPV.138511 (Fig. 12), but no details can be provided.
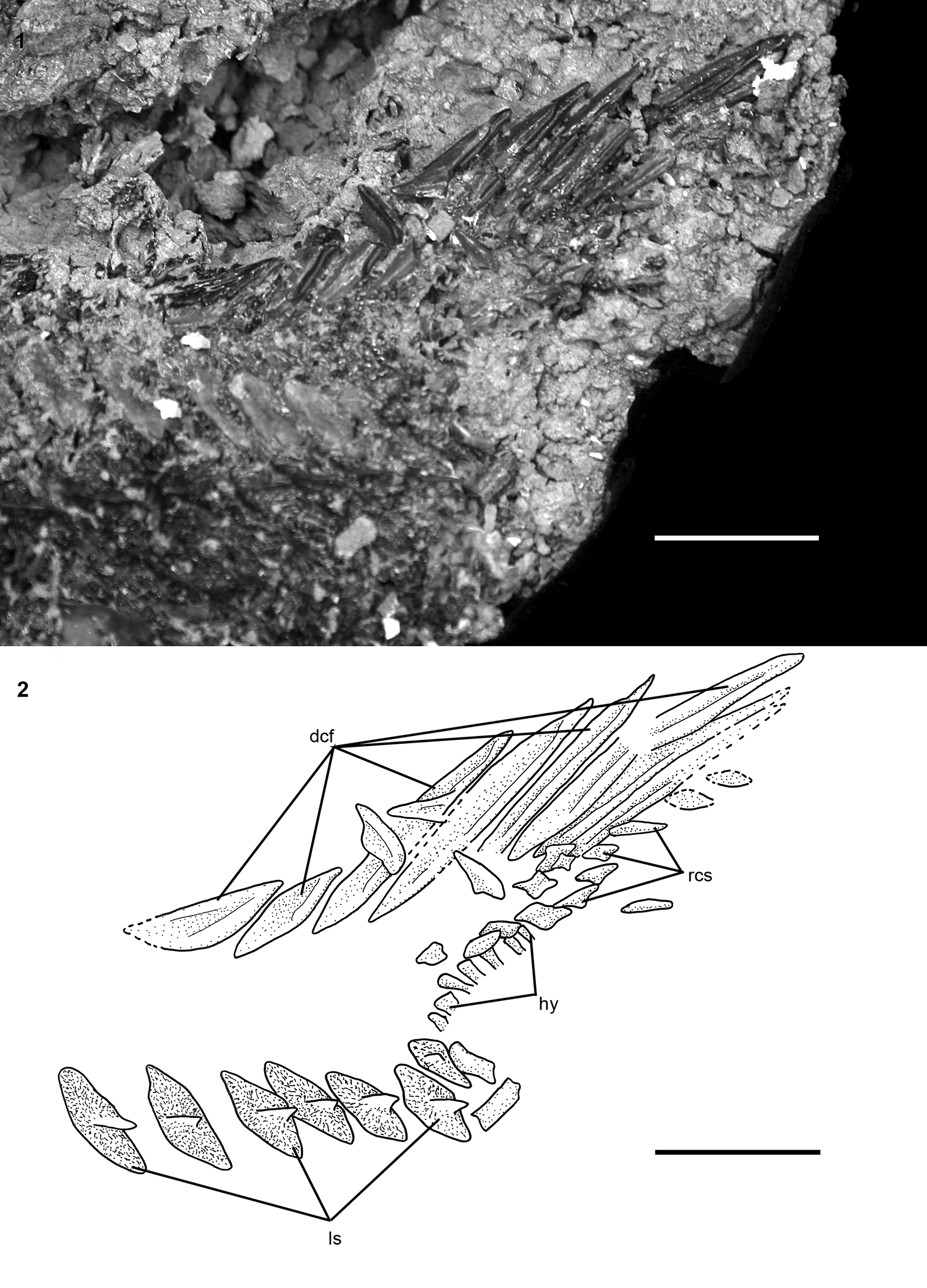
Figure 12. †Acipenser praeparatorum n. sp. (DMNH EPV.138511), caudal peduncle in lateral view. (1) Photograph and (2) line drawing. Anterior facing left. Abbreviations: dcf, dorsal caudal fulcra; hy, hypural; ls, lateral scute; rcs, rhombic caudal scales. Scale bars = 2 cm.
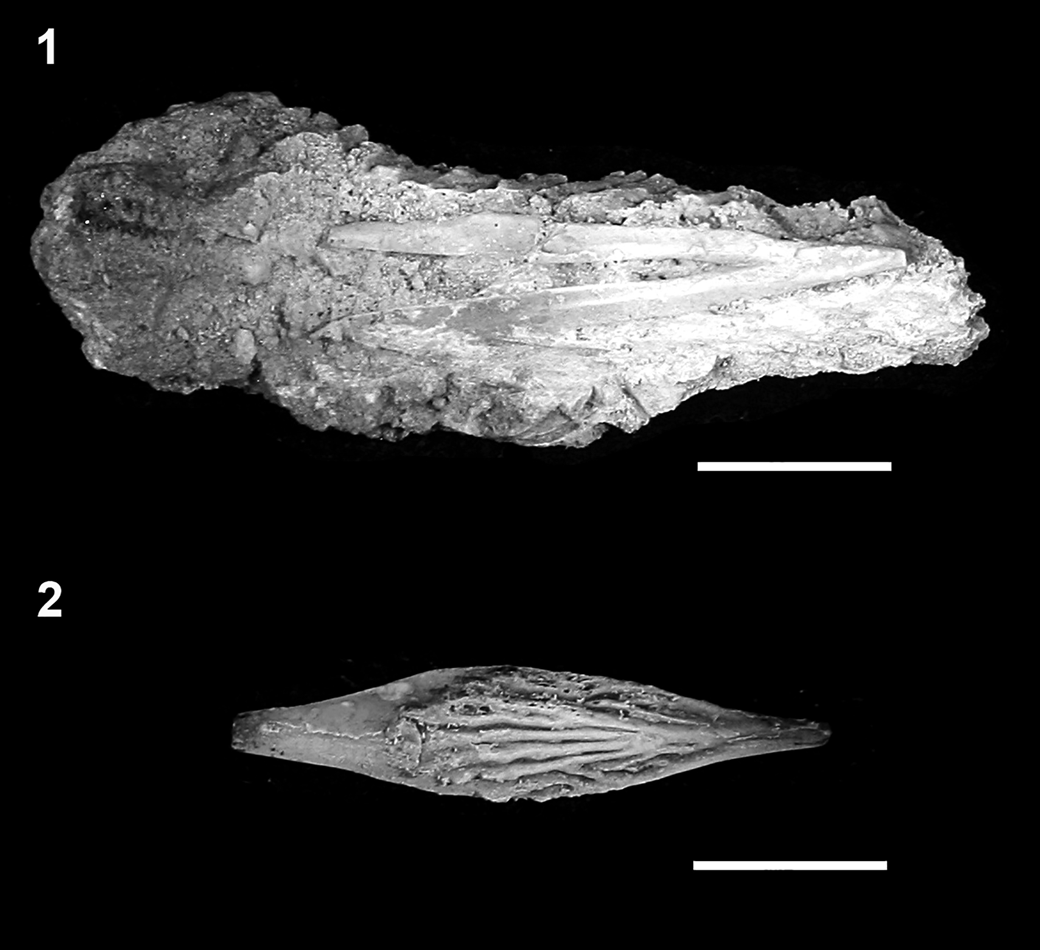
Figure 13. †Acipenser praeparatorum n. sp., isolated fulcral scales in dorsal view. (1) Dorsal caudal fulcrum (FMNH PF17618); (2) first dorsal or ventral caudal fulcrum (FMNH PF17619). Anterior facing left. Scale bars = 1 cm.
Pectoral and pelvic girdles and fins
The bones of the pectoral girdle preserved in our specimens of †Acipenser praeparatorum n. sp. include the posttemporal, supracleithrum, cleithrum, postcleithrum, clavicle, and interclavicle (Figs. 4–7, 14). The pectoral girdle is preserved completely articulated in FMNH PF17608 (Fig. 4), and partially preserved in other specimens. The posttemporal (described above) sutures tightly to the supracleithrum through a broad unornamented sheet of bone extending from the anterior edge of the supracleithrum. This is a similar to extant acipenserids (Hilton et al., Reference Hilton, Grande and Bemis2011). The lateral surface of the supracleithrum ventral to its articulation with the posttemporal is heavily ornamented. The medial surface, seen on a disarticulated specimen (Figs. 8, 9), is smooth dermal bone, marked by a shallow concavity on its ventral portion. Posteroventrally, the supracleithrum contacts the unornamented, leaf-shaped postcleithrum, which is only preserved in FMNH PF17608 (Fig. 4). As in extant sturgeons (Hilton et al., Reference Hilton, Grande and Bemis2011, fig. 26), the cleithrum and clavicle form a so-called cardiac shield that defines the posterior limits of the branchial chamber (Fig. 4); no round-based scales that are found on its surface in extant acipenserids were found in our specimens, but this is likely due to lack of preservation. The tall cleithrum supports the articulation with the pectoral-fin spine. The ventrolateral surface near the contact with the pectoral-fin spine is ornamented. The clavicle is well preserved in the holotype, as well as in an isolated specimen (Fig. 18). The ventral surface of the clavicle is heavily ornamented with radiating lines of ornament and a low, but distinct, ridge that forms into two posteriorly directed spines (Fig. 14). A single isolated element (Fig. 14.7, 14.8) was identified as an interclavicle based on its similarity to that of extant acipenserids (Hilton et al., Reference Hilton, Grande and Bemis2011, fig. 89). This bone is flat, irregularly oval, and has a prominent ridge of ornamentation that in life position would have been exposed to the surface between the left and right clavicles.
The pectoral-fin spine of †Acipenser praeparatorum n. sp. is thick and robust (Fig. 15), and 57–83% head length for the three specimens for which both pectoral-fin spine length and head length could be measured (DMNH EPV.138511, FMNH PF17607, FMNH PF17608). The spine also bears a series of deep grooves between longitudinal ridges. These ridges remain largely separate, but occasionally do anastomose.
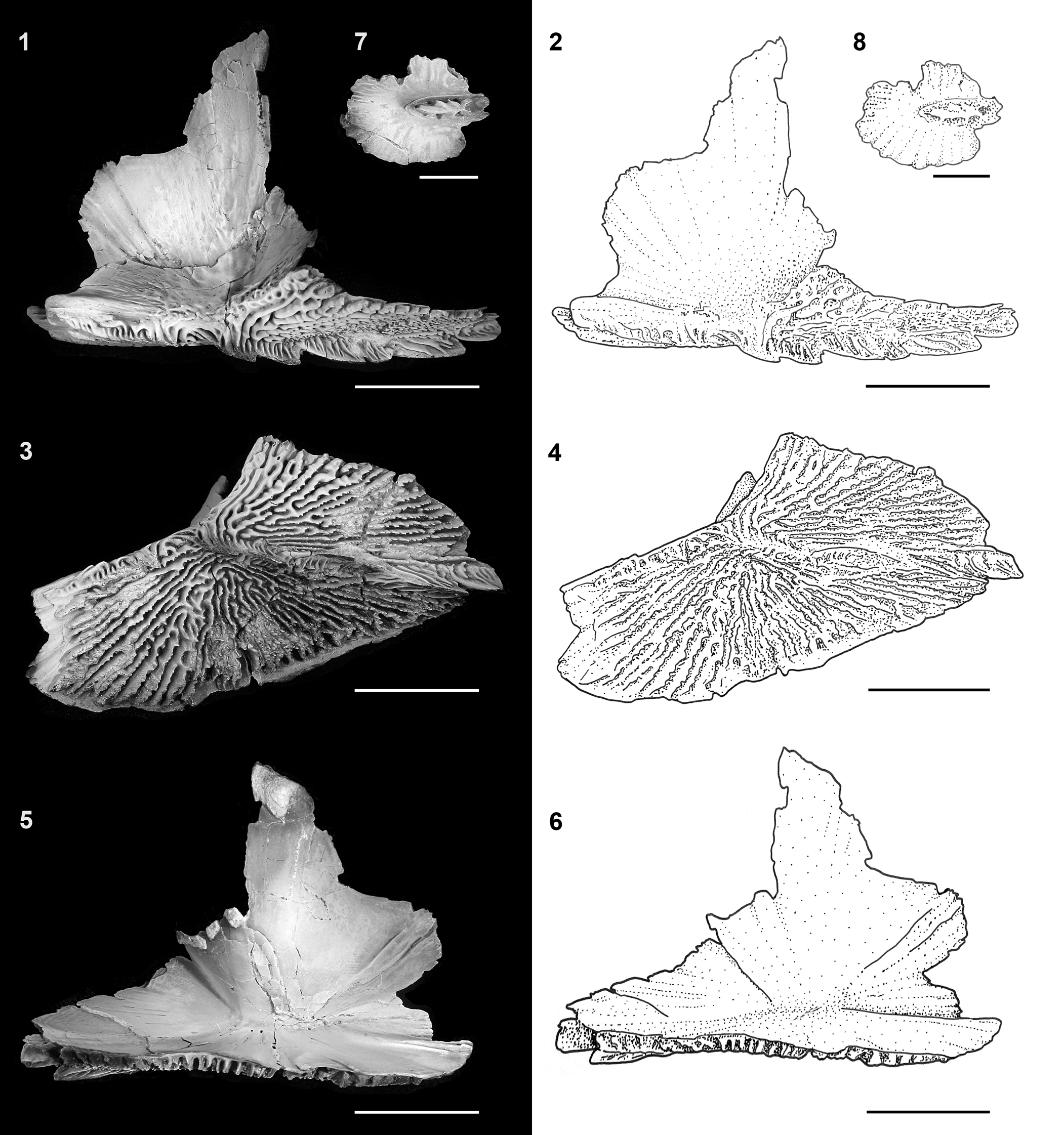
Figure 14. †Acipenser praeparatorum n. sp., isolated bones of the pectoral girdle. (1, 2) Photograph and line drawing of clavicle in lateral view, (3, 4) photograph and line drawing of clavicle in ventral view, and (5, 6) photograph and line drawing of clavicle in medial view (FMNH PF17612). (7, 8) Photograph and line drawing of interclavicle in ventral view (FMNH PF17611). Anterior facing left (5, 6, image reversed so anterior faces left). Scale bars = 2 cm in (1–6), 5 mm in (7, 8).
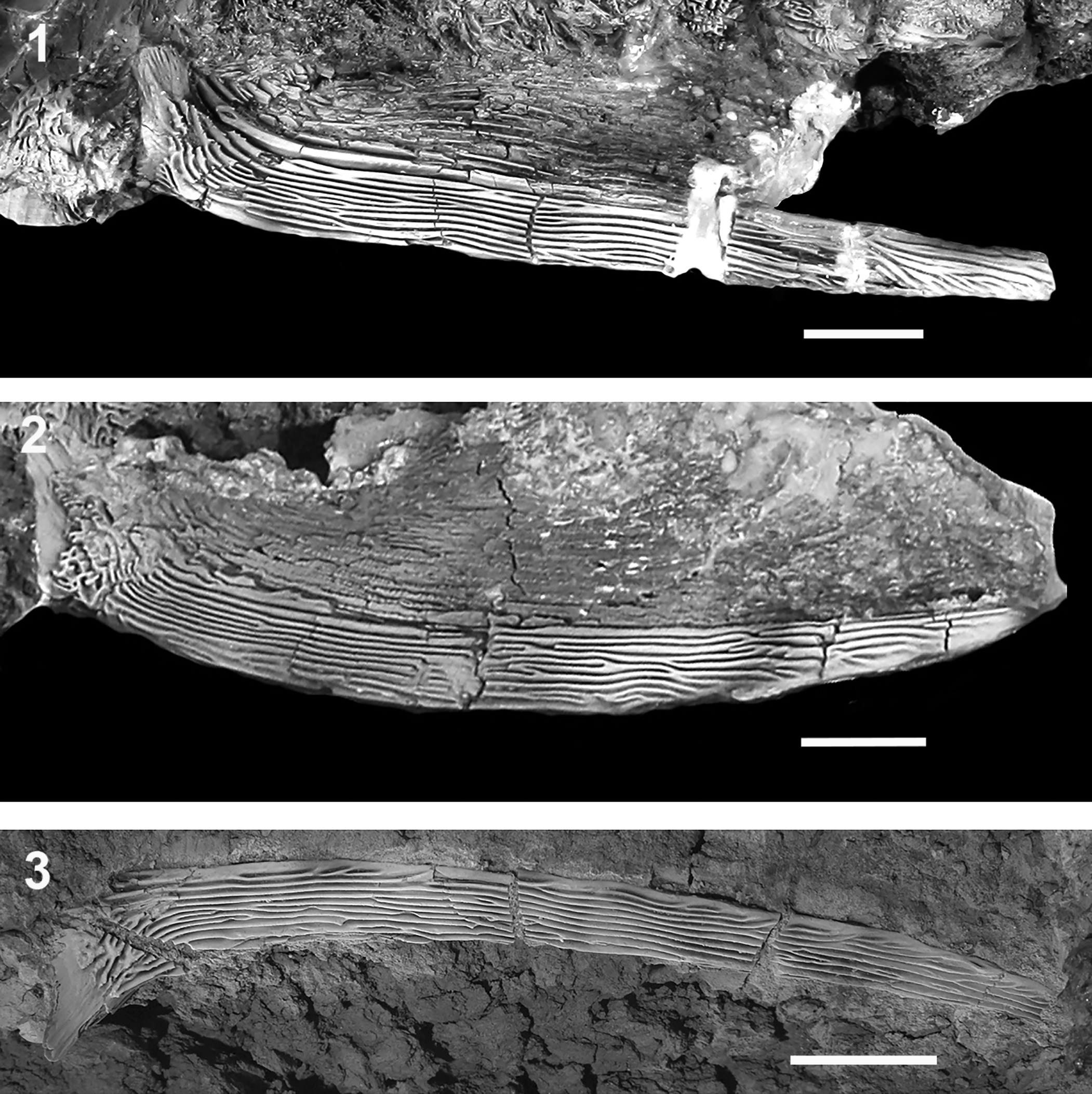
Figure 15. †Acipenser praeparatorum n. sp. pectoral fin spines. Photographs of (1) FMNH PF17608 (holotype), lateral view; (2) FMNH PF17607, lateral view; (3) FMNH PF17624, dorsal view. Anterior facing right (2), image reversed so anterior faces left). Scale bars = 2 cm.
The bases of five and six pelvic-fin rays are preserved in FMNH PF17608 and DMNH EPV.138511, respectively, but they surely numbered greater in life. Bones of the pelvic girdle are not preserved.
Scutes and body scales
The body of †Acipenser praeparatorum n. sp. is armored by five rows of scutes as well as bony scales intercalated between the rows of scutes (Figs. 16, 17). All scutes are heavily ornamented and bear a prominent, sharply pointed ridge, often developed into a well-developed thorn-shaped spine, as seen in several extant species of Acipenseridae (Hilton et al., Reference Hilton, Grande and Fan2021). These spines lack the ornamentation found on the surface of the rest of the scute, but may have fine striations at their base; these striations may extend over much of their surface (Fig. 17.2).
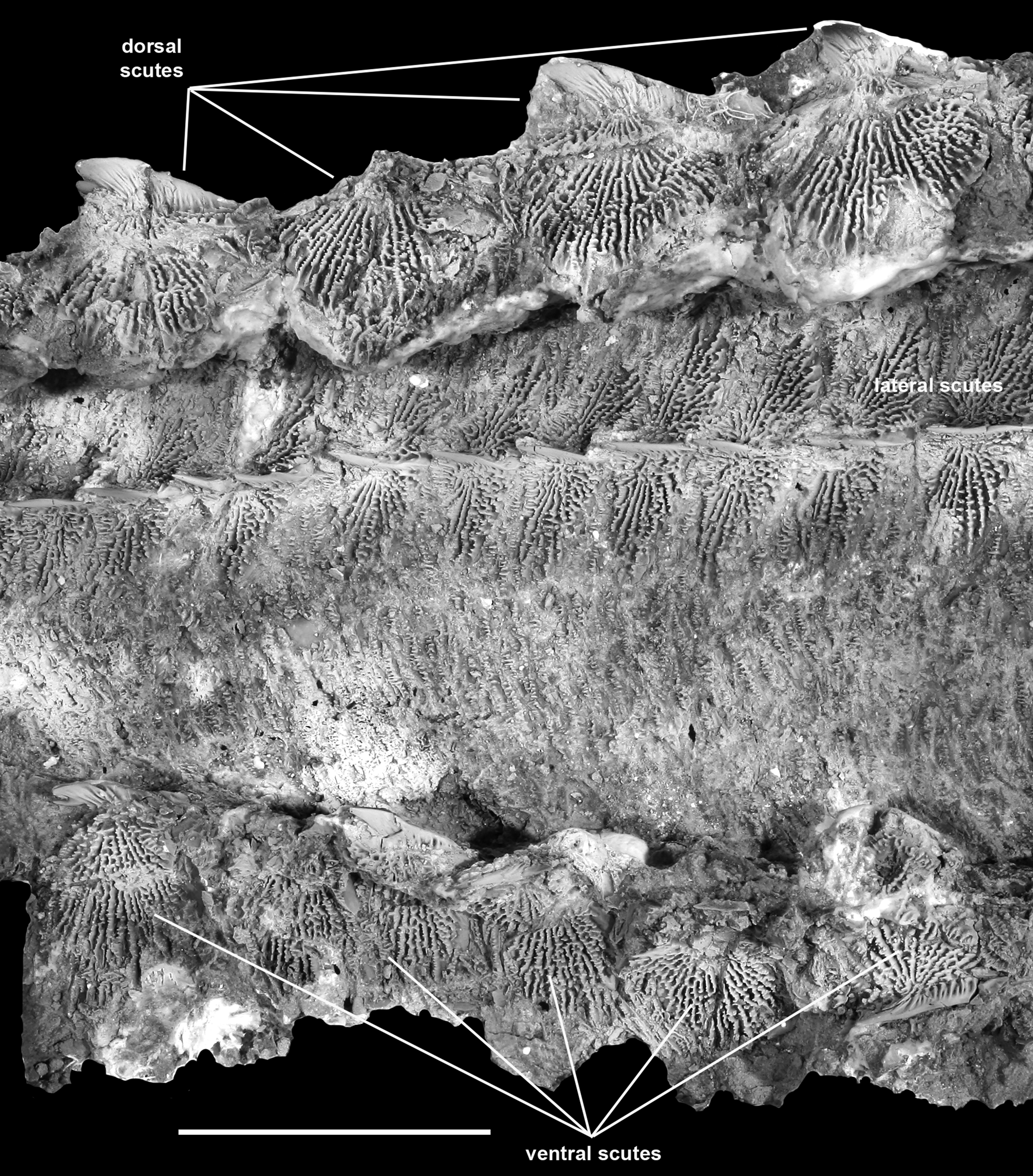
Figure 16. †Acipenser praeparatorum n. sp. (FMNH PF17607), body scutes in situ, lateral view, showing small dermal plates intercalated between scute rows. Anterior facing right. Scale bar = 5 cm.
Figure 17. †Acipenser praeparatorum n. sp., isolated body scutes. (1) Dorsal and (2) lateral views of isolated dorsal scute (FMNH PF17616); (3) lateral view of lateral scute (FMNH PF17615); (4) lateral view of anterior ventral scute (FMNH PF17617). Anterior facing left. Scale bars = 1 cm.
Including the first dorsal scute incorporated into the skull roof, there are 11 dorsal scutes in both FMNH PF17608 and DMNH EPV.138511 (the only two specimens that preserve the entire series). The first dorsal scute is the largest and is fully incorporated into the skull roof. The more posterior scutes are all approximately the same size and appear to be similar in shape to one another.
Only DMNH EPV.138511, with 41 left lateral scutes exposed, preserves the entire series of lateral scutes. However, many lateral scutes can be identified on the other whole-body specimens (Figs. 2, 16) and as isolated specimens (Figs. 8, 9, 17.3). The lateral scutes become progressively smaller from anterior to posterior. All are tall (depth to width ratio of ~39–45%, as measured on multiple scutes from DMNH EPV.138511), with an anterior inclination. The anterior margin of the lateral scutes bears a narrow unornamented region, presumably indicating the position of the overlap from the next anterior scute. The ornamentation of the lateral scutes is formed by a series of ridges of bone radiating from the central ridge or spine anteriorly on the element. On the posterior portion of the scute, both dorsal and ventral to the central ridge, the ornamentation is different, and is in the form of horizontally oriented ridges of bone (Fig. 17.3).
The ventral scutes are the most poorly preserved of all the series of scutes in our specimens. Only the left side of FMNH PF17607 preserves what appears to be a complete series of elements, comprising 11 scutes; the precise shape of the ventral scutes cannot be determined. The scutes appear to be closely set to one another, if not overlapping (Fig. 16). A single isolated specimen (Fig. 17.4) was identified as the anteriormost ventral scute, based on its similarity to that element in extant sturgeons (Hilton et al., Reference Hilton, Grande and Bemis2011, fig. 104), being relatively tall and angular.
Body scales between the scutes (Fig. 16) are tall, overlapping, and irregularly shaped. These cover the body but are most densely packed between the lateral and ventral scute series. The scales are ornamented with horizontally arranged rows of small, tooth-like ornamentation, similar in form to the ornamentation on the posterior portions of the lateral scutes.
Etymology
praeparatorum; after praeparātī (Latin, feminine, genitive, of praeparāre) meaning to “make ready beforehand,” used as a noun in apposition. In recognition of the efforts of the two fossil preparators that worked on these specimens, Connie Van Beek and Kathryn Passaglia of the Field Museum, and more generally to Akiko Shinya and the rest of the fossil preparators at the Field Museum. Without their skill, insight, and determination, many of the details we describe and many of the discoveries we (i.e., paleontologists) make would, quite literally, be buried in rock. Van Beek was the lead preparator on the two whole-body specimens of this new species (as well as the preparator for †Priscosturion longipinnis [Grande and Hilton, Reference Grande and Hilton2006]), and Passaglia was the preparator of the large block that contained an isolated head of this new species, many isolated elements referred to this species, the partial skull belonging to the second new species of acipenserid described below, and two polyodontid skeletons; this large block took ca. 3000 hours to prepare. Other preparators, including those at the DMNH, and volunteers at both FMNH and DMNH are also to be commended for their work.
Remarks
The genus Acipenser cannot be differentially diagnosed within the family Acipenseridae, which also includes the extant genera Huso and Pseudoscaphirhynchus (Birstein et al., Reference Birstein, Doukakis and DeSalle2002; Hilton et al., Reference Hilton, Grande and Bemis2011). However, until a robust phylogenetic analysis including all putative fossil and living species is performed, which is beyond the scope of this paper, and which can be used as the basis for a taxonomic review of all species of Acipenseridae, we choose to use the genus name Acipenser in its general sense (i.e., Acipenser sensu Bemis et al., Reference Bemis, Findeis and Grande1997) to classify these new species of Acipenseridae rather than establish a new genus-group name for the fossils described herein. Much further work is yet needed to satisfactorily resolve the detailed interrelationships of fossil and living acipenserids.
†Acipenser amnisinferos new species
Figures 18–20
Holotype
FMNH PF17629 (Figs. 18–20), skull, pectoral girdle, anterior scutes (crushed and partially disarticulated) exposed in left lateral view.
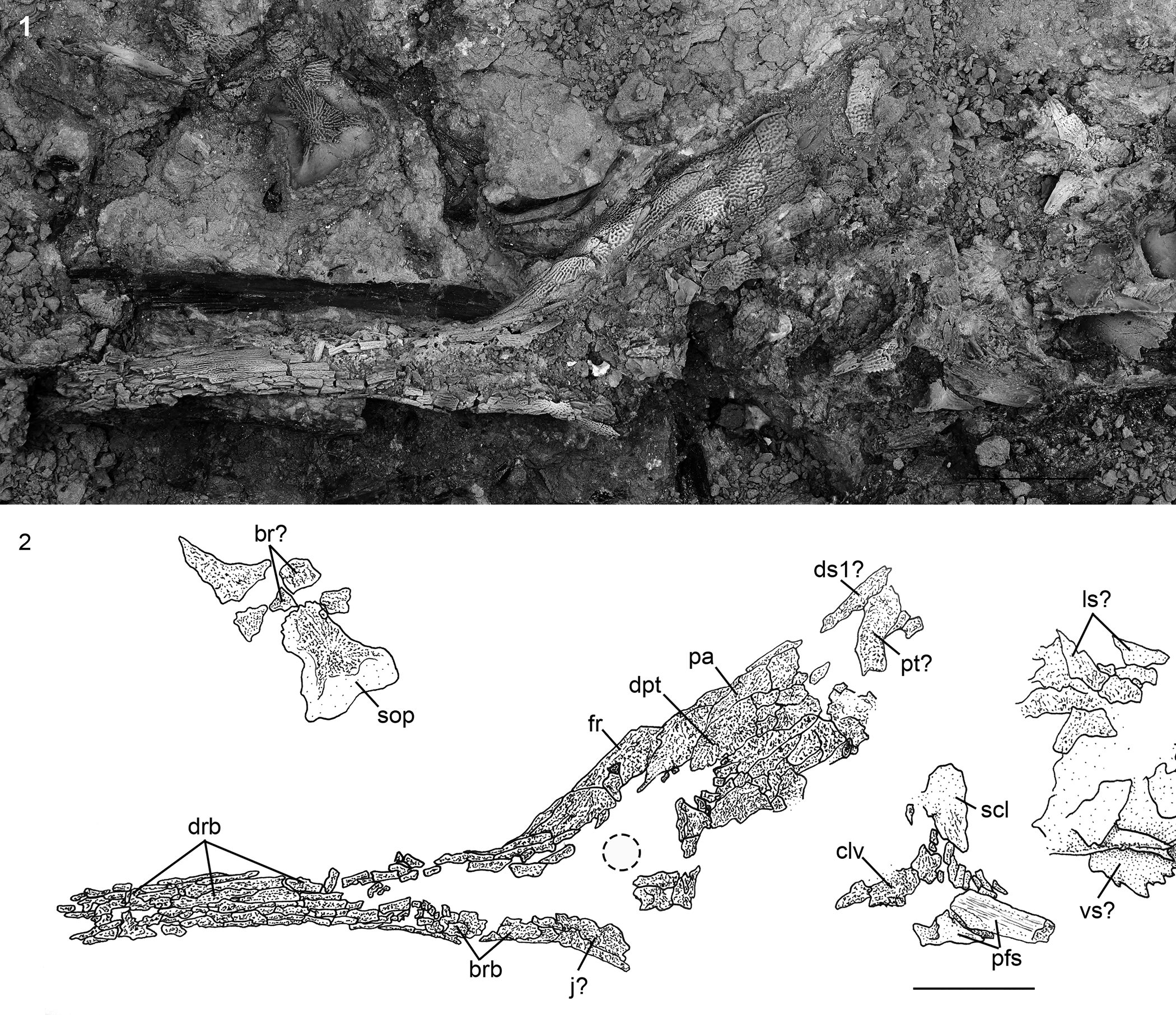
Figure 18. †Acipenser amnisinferos n. sp. (FMNH PF17629, holotype), skull and shoulder girdle in left lateral view. (1) Photograph and (2) line drawing; dashed line circle indicates estimated position of the eye. Anterior facing left. Abbreviations: br, branchiostegal; brb, border rostral bones; clv, clavicle; dpt, dermopterotic; drb, dorsal rostral bone; ds1, first dorsal scute; fr, frontal; j, jugal; ls, lateral scute; pa, parietal; pfs, pectoral-fin spine; pt, posttemporal; scl, supracleithrum; sop, subopercle; vs, ventral scute. Scale bars = 5 cm.
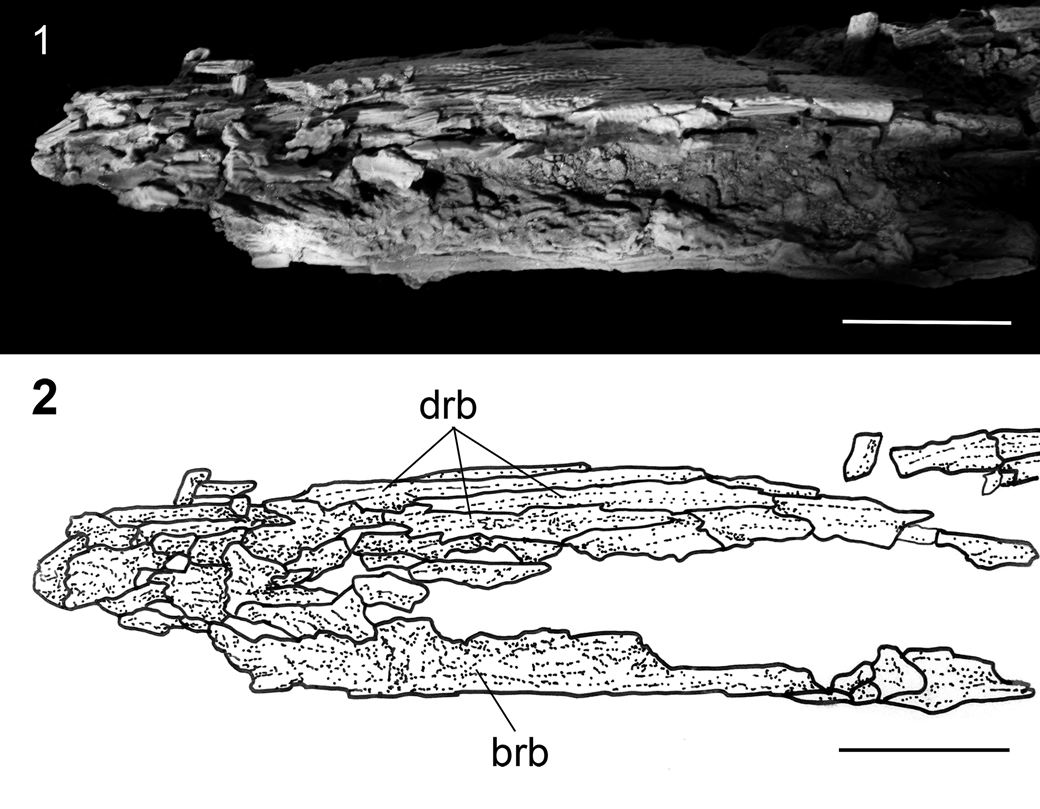
Figure 19. †Acipenser amnisinferos n. sp. (FMNH PF17629, holotype), anterior tip of rostrum in ventrolateral view. (1) Photograph and (2) line drawing. Anterior facing left. Scale bars = 2 cm. Abbreviations: brb, border rostral bones; drb, dorsal rostral bone.

Figure 20. †Acipenser amnisinferos n. sp. (FMNH PF17629, holotype), showing detail of the ornamentation of the dermal bones. Anterior facing left. Scale bar = 5 cm.
Diagnosis
An acipenserid that differs from all other known species by an elongate snout (~57% head length). The only other sturgeon taxon with a snout of this relative length is the extant species Acipenser stellatus from the Black and Caspian seas and their tributaries, which has a snout 51–68% head length (Hilton et al., Reference Hilton, Dillman, Paraschiv and Suciu2022). Although these two taxa overlap in relative snout lengths, adult or large subadult specimens of A. stellatus (i.e., >1 m standard length) have a snout length that is an average of 61% head length (n = 38; Hilton, unpublished data). Also, the dorsal dermal skull bones of A. stellatus bear small ridges or thorns at their centers of origins; these are lacking in †A. amnisinferos n. sp., and there is no indication that this is due to preservation (e.g., broken bases of spines; see Hilton et al., Reference Hilton, Dillman, Paraschiv and Suciu2022, figs. 5–7 for condition in A. stellatus).
Occurrence
Tanis site (DePalma et al., Reference DePalma, Smit, Burnham, Kuiper and Manning2019) of the Hell Creek Formation, Bowman County, North Dakota, U.S.A.; Late Cretaceous (Maastrichtian); type specimens collected by Steve Nicklas and Robert Coleman in 2010.
Description
Snout elongate, 57% head length; ornamentation of the skull roofing bones and other dermal bones consisting of flattened ridges of bones forming a network of cells; snout is covered by elongate, narrow dorsal rostral bones and border rostral bones; posterior outline of subopercle uncertain, but bears substantial unornamented region along anterior and dorsal margin with strongly ornamented central portion; fragments of bones tentatively identified as branchiostegals are suggestive that these are flattened elements; fragments of at least 12 scutes preserved, but no details available.
Anatomical description
In this section, we provide a detailed description of †Acipenser amnisinferos n. sp. organized by region of the body.
Dermal bones of the skull roof
The skull bones of the unique specimen are crushed and few details of the sutures and relationships among different elements can be discerned. Elements that are preserved include the frontals, parietals, dermopterotics, and possibly the jugal, the first dorsal scute, and the posttemporal, but none can be described (Fig. 18). Anteriorly, the snout is covered by elongate, narrow dorsal rostral bones and border rostral bones (Figs. 18, 19). The snout is long (preorbital length is ~200 mm) and is 57% of the estimated head length.
Most notably, the ornamentation of the skull roofing bones and other dermal bones preserved (opercular series, pectoral girdle, and scutes) in †A. amnisinferos n. sp. is in marked contrast to that of †A. praeparatorum n. sp. Rather than well-defined radiating ridges of bone, the ornamentation of †A. amnisinferos n. sp. is flattened and in the form of a series of cells (Fig. 20).
Opercular series
The subopercle is the only element of the opercular series that can be identified (Fig. 18). The portion of the subopercle that is preserved bears ornamentation similar to the skull roofing bones, as well as large unornamented areas anteriorly and dorsally. The posterior margin of the subopercle is broken, so the precise shape is unclear.
There are two fragments of bones that may be part of the branchiostegals (Fig. 18), although this identification is uncertain. If correct, though, the branchiostegals are flattened dermal elements, similar to the ventral branchiostegals of extant species (e.g., Hilton et al., Reference Hilton, Grande and Bemis2011, fig. 48).
Pectoral girdle and fin spine
The poorly preserved pectoral girdle is is represented by crushed and fragmentary portions of the left posttemporal, supracleithrum, cleithrum, clavicle, and pectoral-fin spine (Fig. 18). Only the base of the pectoral-fin spine is preserved, so no details can be described.
Scutes
There are fragments of at least 12 scutes preserved (Fig. 18). Although no details are preserved beyond the similar ornamentation to the other dermal bones, and it is unclear how the elements were organized in life, it appears that the first dorsal scute, lateral scutes, and ventral scutes are all represented in this specimen (Fig. 18).
Etymology
From Latin; a combination of amnis, meaning creek or stream, and inferos, meaning Hell, in reference to the Hell Creek Formation in which this species was discovered; nouns used in apposition.
Referred specimen
A second specimen (FMNH PF17620), found and removed from the same block that contained the holotype, may represent a second specimen and can be referred to the new species based on similar pattern of ornamentation on the exposed dermal bones. The specimen comprises portions of the skull roof and pectoral girdle (Fig. 21.1). Also preserved on this specimen are fragments of the parasphenoid, thirteen ribs (Fig. 21.2). An associated specimen (FMNH PF17621; Fig. 22) comprises a pectoral-fin spine and fin rays. This associated pectoral fin bears a fin spine that appears to be more slender than that of †Acipenser praeparatorum n. sp., although this may be an ontogenetic artefact. If this specimen is †Acipenser amnisinferos n. sp., it represents a significantly smaller individual than the holotype. However, we have left its identification as †Acipenser cf. A. amnisinferos n. sp.
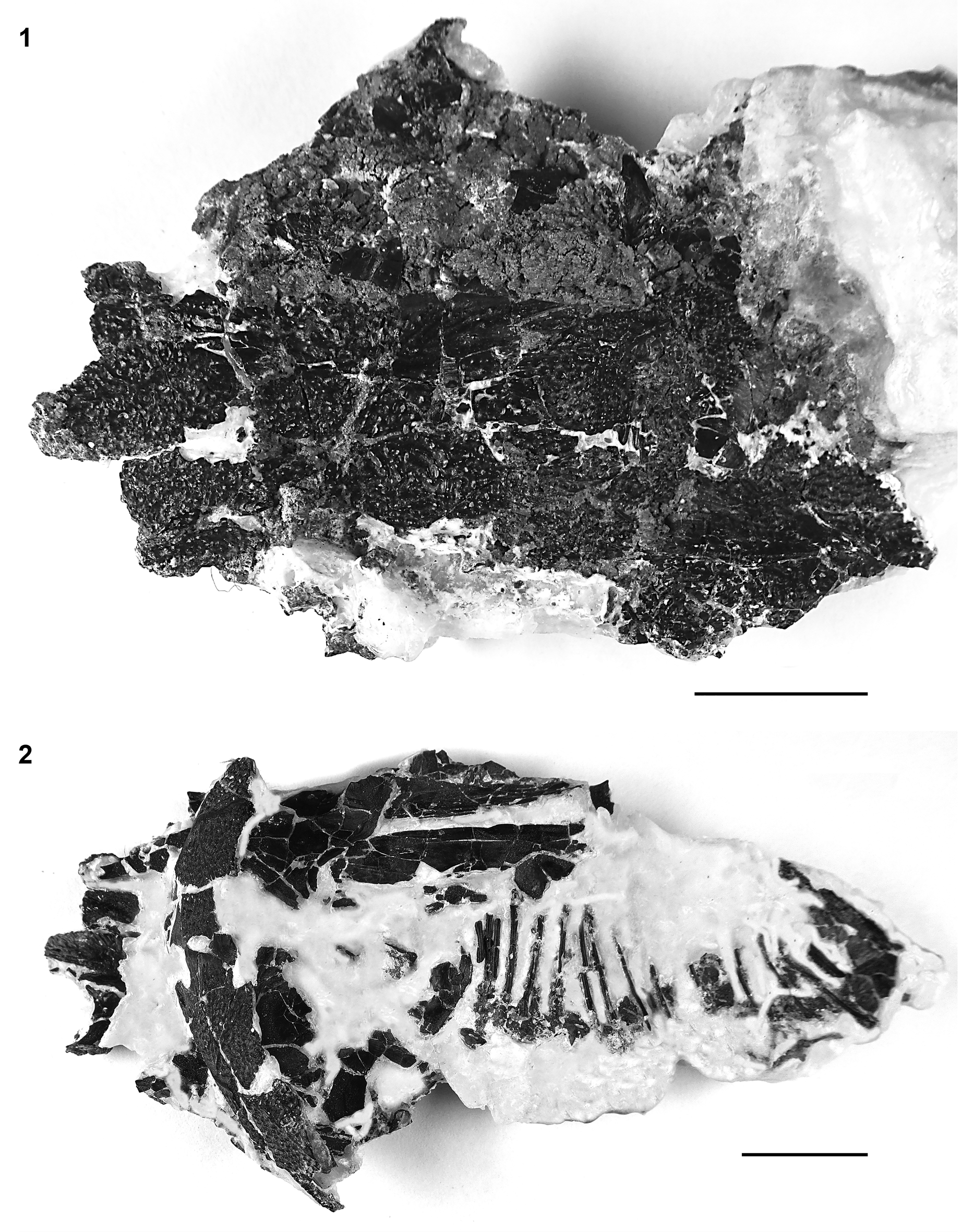
Figure 21. †Acipenser cf. A. amnisinferos n. sp. (FMNH PF17620), skull roof, pectoral girdle, and ribs. (1) Dorsal and (2) ventral views. Anterior facing left. Scale bars = 2 cm.
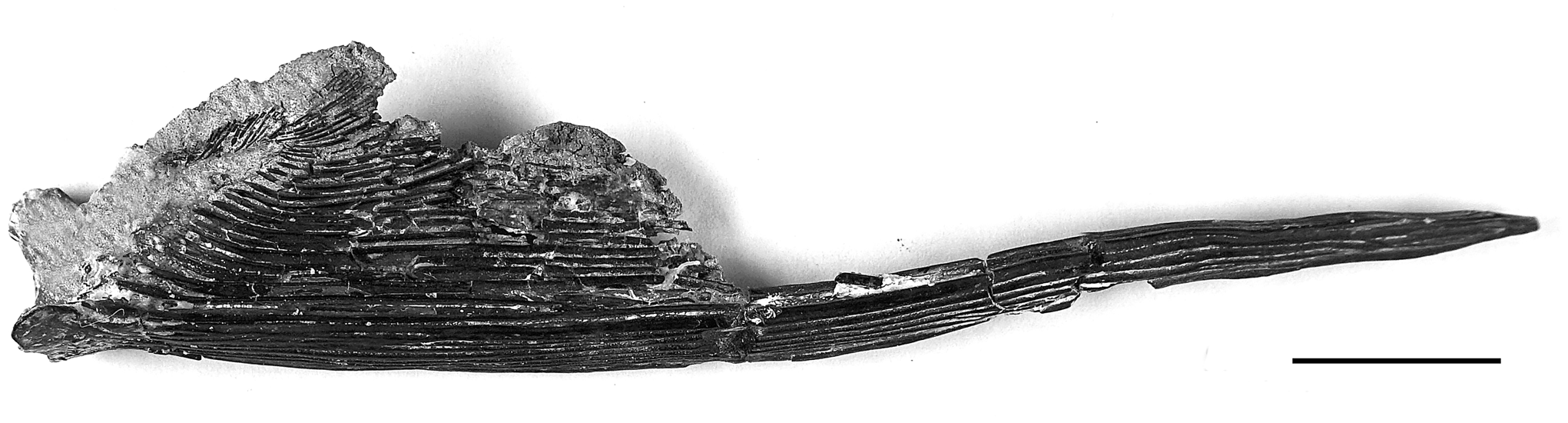
Figure 22. †Acipenser cf. A. amnisinferos n. sp. (FMNH PF17621), pectoral and spine, dorsal view. Anterior facing left. Scale bar = 2 cm.
Remarks
The skeleton of the single specimen of †A. amnisinferos n. sp. is very poorly preserved and is represented only by a partial skull (comprising dermal bones of the skull roof, snout, and opercular series), the base of the pectoral fin spine, and fragments of the dermal pectoral girdle, and isolated and displaced scutes. Nevertheless, preserved portions of the skeleton are sufficiently complete to differentially diagnose it from other sturgeon taxa. A more complete description, however, awaits the discovery of additional and more complete specimens.
Brief review of other Cretaceous Acipenseridae from North America
In this section we briefly review and comment on other Cretaceous Acipenseridae from North America, including nominal taxa and other notable occurrences, to provide materials for the reassessment of the phylogenetic relationships among fossil and living members of the family.
†Protoscaphirhynchus Wilimovsky, Reference Wilimovsky1956
Figure 23
Type species
†Prostoscaphirhynchus squamosus Wilimovsky, Reference Wilimovsky1956, by monotypy.
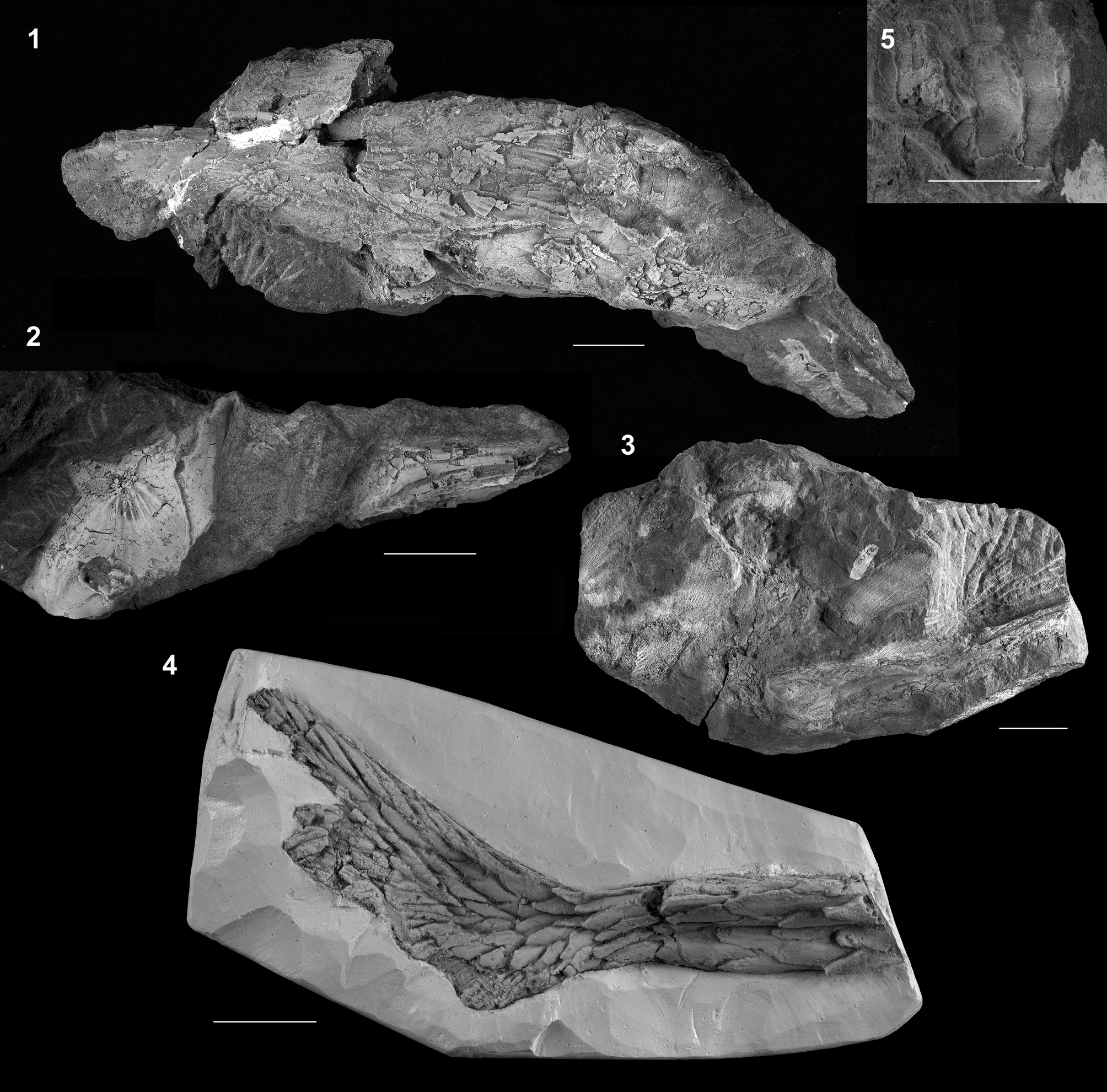
Figure 23. †Protoscaphirhynchus squamosus Wilimovsky, Reference Wilimovsky1956 (UMMP 22210, holotype). (1) Skull roof in dorsal view; anterior facing left; (2) dorsal scutes; anterior facing left; (3) other side of the block with the skull roof, showing the ventral surface of the left clavicle and the proximal portion of the left fin spine; anterior facing left; (4) posterior portion of body and portions of paired fins; (5) cast of the caudal fin, anterior facing right. Scale bars = 2 cm (1–3, 5) and 5 cm (4). Modified from Hilton and Grande (Reference Hilton and Grande2006, figs. 1, 2).
Holotype
UMMP 22210; portions of the skull, pectoral girdle, body, and caudal fin.
Occurrence
Hell Creek Formation; “about 24 miles SE of Fort Peck Montana” (Wilimovsky, Reference Wilimovsky1956, p. 1207); Late Cretaceous.
Remarks
†Prostoscaphirhynchus squamosus is known from a single poorly preserved and poorly prepared specimen from the Hell Creek Formation of Montana. The specimen includes portions of the skull, pectoral girdle, scutes, body, and caudal fin (Fig. 23). The caudal peduncle is of note, because it is flattened and entirely encased in rows of scutes, similar to that of Scaphirhynchus. †Prostoscaphirhynchus squamosus was redescribed by Hilton and Grande, (Reference Hilton and Grande2006).
†Priscosturion Grande and Hilton, Reference Grande and Hilton2009
Figure 24
Type species
†Psammorhynchus longipinnis Grande and Hilton, Reference Grande and Hilton2006, by monotypy; Grande and Hilton (Reference Grande and Hilton2009) provided a genus replacement name, †Priscosturion, for the species, because Psammorhynchus was preoccupied in flatworms.
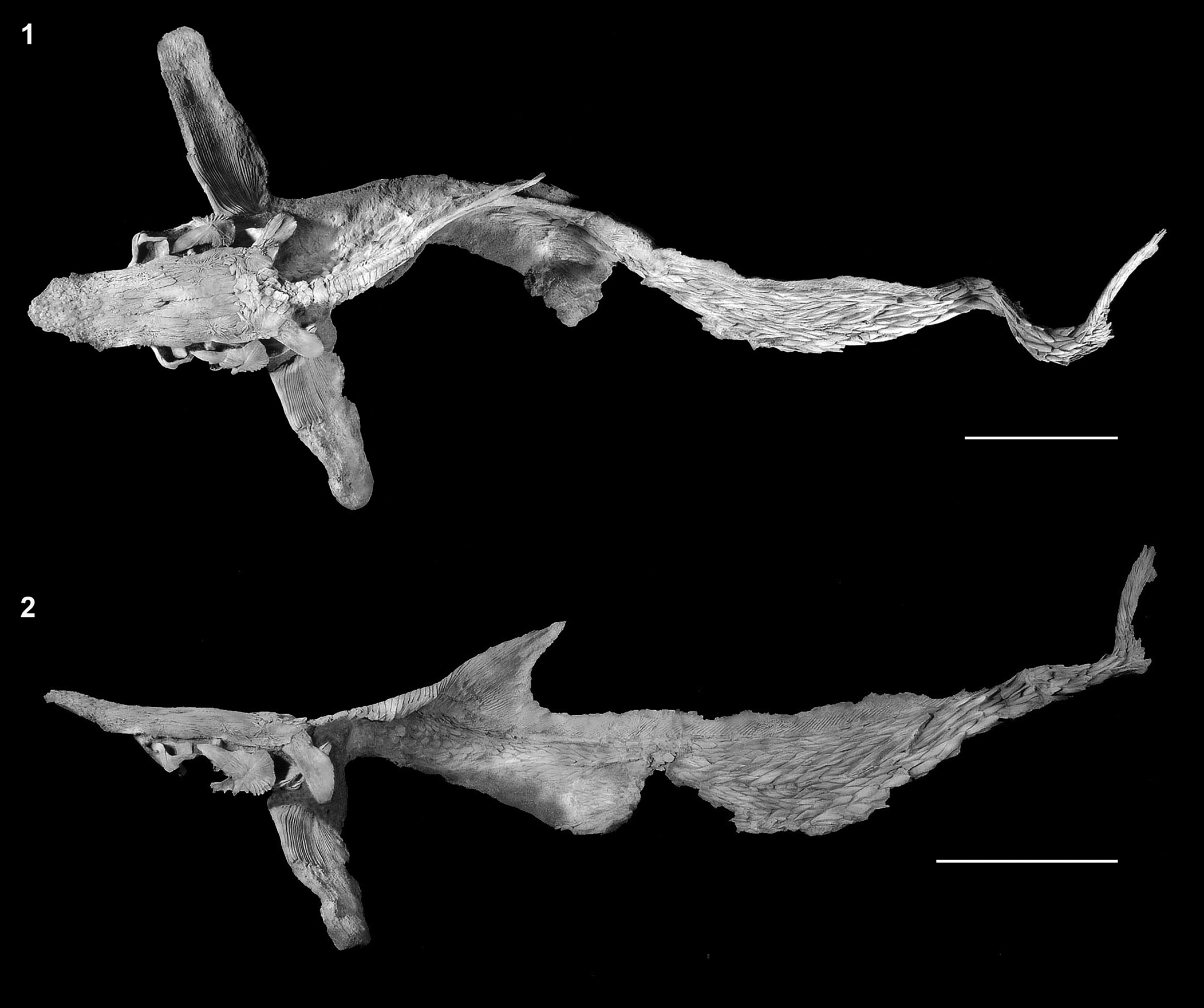
Figure 24. †Priscosturion longipinnis (Grande and Hilton, Reference Grande and Hilton2006), MOR 1184, holotype, entire skeleton in (1) dorsal and (2) lateral views. Anterior facing left. Modified from Grande and Hilton (Reference Grande and Hilton2006, figs. 3, 4.1, respectively).
Holotype
MOR 1184; whole body specimen comprising skull, pectoral girdle and fin, dorsal fin, body, and a portion of the caudal fin.
Occurrence
Judith River Formation of Montana, Late Cretaceous.
Remarks
The osteology of †Priscosturion longipinnis was described in detail by Grande and Hilton (Reference Grande and Hilton2006). Notable features of this species include the presence of only the dorsal scute series, the presence of elongate, rhomboid body scales, thickened rectangular lateral line scales, and an elongate dorsal fin that spans ~80% of the body length (Fig. 24). Hilton et al. (Reference Hilton, Grande and Bemis2011) recovered †Priscosturion longipinnis as the sister group of all other sturgeons, or as the sister group of Scaphirhynchus.
†Anchiacipenser Sato et al., Reference Sato, Murray, Vernygora and Currie2018
Figure 25
Type species
†Anchiacipenser acanthaspis Sato et al., Reference Sato, Murray, Vernygora and Currie2018, by monotypy.

Figure 25. †Anchiacipenser acanthaspis Sato et al., Reference Sato, Murray, Vernygora and Currie2018, UALVP 56596, holotype. Anterior facing left. Scale bar = 5 cm. Photo courtesy of Alison Murray.
Holotype
UALVP 56596, skull, pectoral girdle, and body anterior to dorsal fin; preserved portion measures 69 cm.
Occurrence
Dinosaur Park Formation of Alberta, Canada, Late Cretaceous.
Remarks
Sato et al. (Reference Sato, Murray, Vernygora and Currie2018) listed several characters as diagnostic for this genus and species, including: a narrow, pointed rostrum (35–50% HL); dorsal scutes with large lateral wings, radiating ornamentation, and strong median spines; lateral scutes in broad contact with one another and more than three times tall as wide; an “expanded dorsal shield” on the snout formed by dorsal rostral bone; and the presence of border rostral bones. Further, Sato et al. (Reference Sato, Murray, Vernygora and Currie2018) described this species possessing three small square or ovoid branchiostegals. We note that the presence of well-developed median spines on dorsal scutes is a feature found throughout Acipenseridae. Also, the presence of border rostral bones is a feature found throughout the family. It is unclear what aspects of the dorsal rostral bones were diagnostic, because all acipenserids bear dorsal rostral bones forming a “shield” on the rostrum, and from their figure (Sato et al., Reference Sato, Murray, Vernygora and Currie2018, fig. 2), the pattern of these elements does not appear to be distinct from that of other sturgeons.
†Engdahlichthys Murray et al., Reference Murray, Brinkman, Demar and Wilson2020
Type species
†Engdahlichthys milviaegis Murray et al., Reference Murray, Brinkman, Demar and Wilson2020, by monotypy.
Holotype
UWBM 109829; poorly preserved partial skull, anterior portion of body, and associated caudal and anal fins.
Occurrence
Lower Paleocene, Tullock Member of Fort Union Formation of Montana.
Remarks
Although not from the Cretaceous, we include this taxon herein because of the continuity of the Tullock Member of the Fort Union Formation with the Late Cretaceous Hell Creek Formation (Hartman et al., Reference Hartman, Butler, Weiler and Schumaker2014). The skull of the single specimen is poorly preserved (Murray et al., Reference Murray, Brinkman, Demar and Wilson2020, figs. 6–8), and additional material is necessary before anything can be added to its initial description.
Other nominal and noteworthy sturgeons from the Cretaceous of North America Figures 26, 27
The fossil record of Acipenseridae in North America was reviewed by Hilton and Grande (Reference Hilton and Grande2006). Within the Late Cretaceous, Acipenseridae are known from the Milk River Formation (Alberta), the Judith River Group (Alberta, Saskatchewan, and Montana), Prince Creek Formation (Alaska), the Lance Formation (Wyoming), and the Hell Creek Formation (Montana and North Dakota), spanning from the Santonian to late Maastrichtian, in primarily freshwater deposits (only the Judith River Group includes estuarine portions). Among these records, there are two nominal taxa: †Acipenser albertensis Lambe, Reference Lambe1902 (Fig. 26) and †Acipenser eruciferus (Cope, Reference Cope1876) (Fig. 27.1, 27.2). These taxa, as well as most other remains (e.g., Fig. 27.3–27.6), were assigned to Acipenseridae indeterminate genus and species by Hilton and Grande (Reference Hilton and Grande2006), including a specimen referred to †Anchiacipenser acanthaspis by Sato et al. (Reference Sato, Murray, Vernygora and Currie2018) from the Hell Creek Formation (Fig. 27.6).

Figure 26. †Acipenser albertensis Lambe, Reference Lambe1902, CMN 1677, holotype, reproduced from Lambe (Reference Lambe1902, pl. 21, fig. 9). This figure was originally published at the natural size of the specimen; it is enlarged in this reproduction (original is, as positioned on the page, 63 mm tall). Anterior facing left. From Hilton and Grande (Reference Hilton and Grande2006, fig. 3).
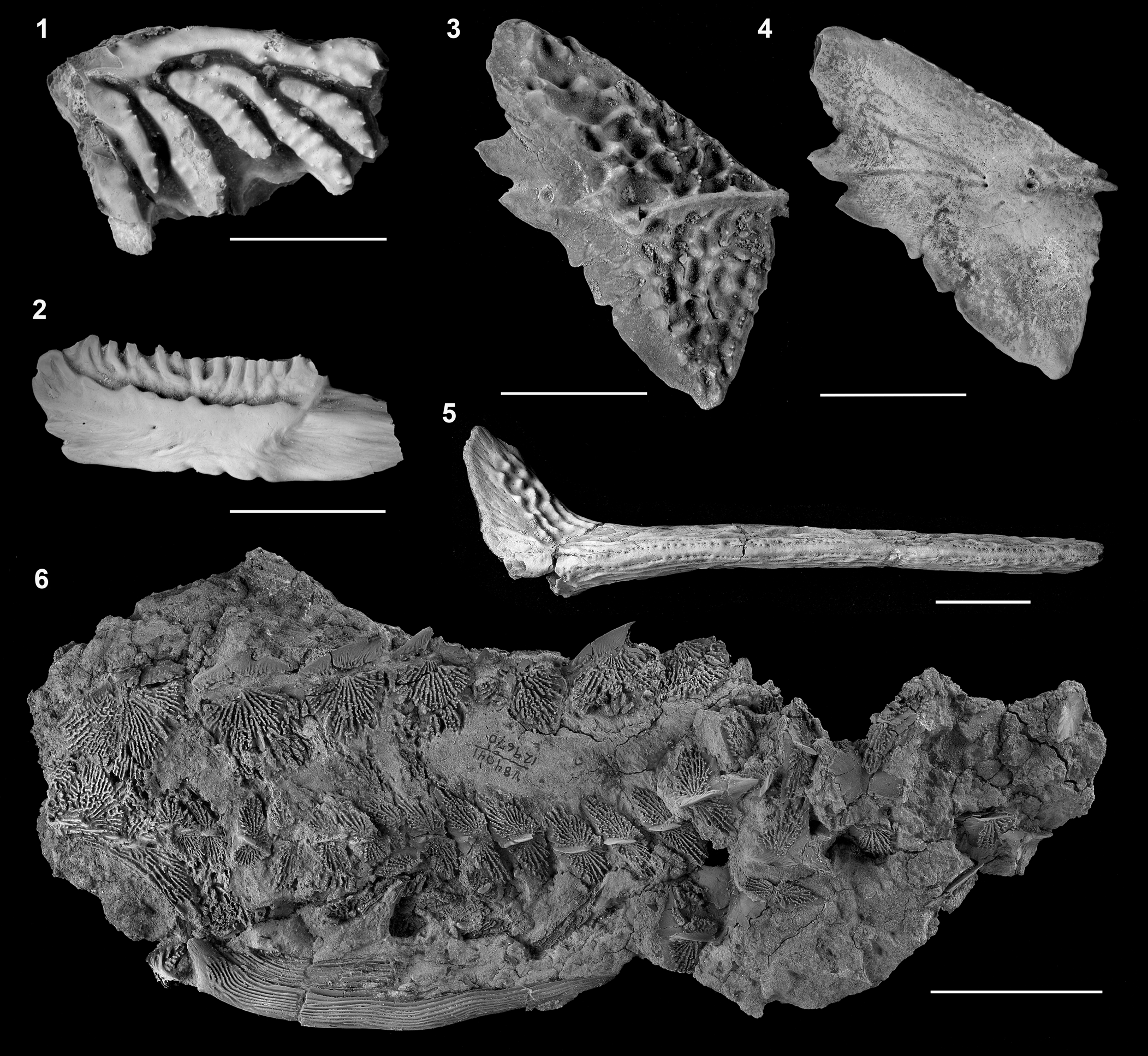
Figure 27. Acipenseridae indeterminate from Hell Creek Formation. (1) †Acipenser eruciferus (Cope, Reference Cope1876), holotype (AMNH 7081); external view, orientation uncertain. (2) †Ceratodus hieroglyphus Cope, Reference Cope1876, holotype (AMNH 7080); considered also to be †A. eruciferus by Estes (1964); view and orientation uncertain. (3) Isolated lateral scute in lateral view (image reversed so that anterior facing left) and (4) medial view. (5) Isolated pectoral fin spine (UCMP 115962). (6) Pectoral girdle and anterior portion of the trunk (UCMP 129670), referred to †Anchiacipenser acanthaspis by Sato et al. (Reference Sato, Murray, Vernygora and Currie2018). Modified from Hilton and Grande (Reference Hilton and Grande2006, figs. 4, 6). Anterior facing left unless otherwise noted. Scale bars = 5 mm (1, 2), 2 cm (3–5), and 5 cm (6).
Discussion
The Cretaceous was a time of great evolutionary transition for the freshwater fish fauna of North America. However, the Cretaceous record of freshwater fishes from North America is notoriously poor, consisting mostly of fragments or isolated skeletal elements (e.g., vertebrae). Rare exceptions include the acipenseriforms discussed in this paper and a some exceedingly rare teleosts (Grande, Reference Grande1986). Specimens representing the two new species of acipenserid described above, and at least one undescribed polyodontid that is currently under study (Hilton, During, Ahlberg, and Grande, in progress), were recovered from the Tanis site. The Tanis site has been implicated as having been deposited in the Cretaceous Tanis River immediately following the Chicxulub impact (DePalma et al., Reference DePalma, Smit, Burnham, Kuiper and Manning2019). Impact spherules were found inside the gill chamber of acipenseriform fishes, specifically associated with gill rakers, but not outside individuals, suggesting that the fishes were actively feeding at the time of the impact and subsequent seiche waves (DePalma et al., Reference DePalma, Smit, Burnham, Kuiper and Manning2019, fig. 6). During et al. (Reference During, Smit, Voeten, Berruyer, Tafforeau, Sanchez, Stein, Verdegaal-Warmerdam and van der Lubbe2022) used carbon isotope analysis, paleohistology, and propagation phase-contrast synchrotron radiation micro-computed tomography to infer seasonal and growth patterns of polyodontid and acipenserid specimens, and concluded that the seiche waves and subsequent inundation of the fishes occurred during the boreal spring. The presence of both marine and freshwater organisms indicates that this site was in close proximity to the shoreline of the Western Interior Seaway.
The two new species of Acipenser described herein add to the taxonomic and morphological diversity of Acipenseridae known from the Late Cretaceous of North America. Five diagnosable species of Acipenseridae are now known, three of which are known from the Hell Creek Formation. These species represent different morphological aspects of acipenserid diversity, including so-called typical sturgeon body plan (†Acipenser praeparatorum n. sp.; †A. amnisinferos n. sp.; †Anchiacipenser acanthaspis), a shovelnose-like sturgeon (†Prostoscaphirhynchus squamosus), and novel combinations of traits (†Priscosturion longipinnis). From this, it is clear that the modern “sturgeon body plan” was established by the Late Cretaceous, although this bauplan had not become firmly set. It is likely that additional morphological and taxonomic diversity will be uncovered as new material is discovered and prepared.
The characteristics of the two species described herein suggest affinities to other species of Acipenseridae. †Acipenser praeparatorum n. sp. has a distinctly enlarged first dorsal scute that spans the width of the skull roof (Fig. 6) and gives the lateral profile a “hunch back” appearance (best seen in Fig. 2.1, 2.2). A similarly enlarged first dorsal scute is seen only in two extant species of Acipenseridae: Acipenser schrenkii Brandt, Reference Brandt1869, from the Amur River (Asia) and A. nudiventris Lovetsky, Reference Lovetsky1828, from the Aral, Caspian, and Black seas (Eurasian). The anatomy of these extant species needs to be better studied to allow for more detailed comparisons to †A. praeparatorum n. sp. However, current phylogenetic hypotheses for the family based on genetic data (e.g., Krieger et al., Reference Krieger, Hett, Fuerst, Artyukhin and Ludwig2008) suggest that the extant taxa are in two distinct clades of Acipenser, with A. schrenkii being included in a trans-Pacific clade as sister to A. transmontanus, and A. nudiventris being the sister group of A. stellatus + Pseudoscaphirhynchus, with more European affinities. Further study of the anatomy of A. schrenkii and A. nudiventris may clarify the affinity of †A. praeparatorum n. sp. and help resolve either an Asian or European biogeographic connection.
The anatomy of †Acipenser amnisinferos n. sp. is mostly unknown due to the incomplete preservation of the unique specimen. However, based on the portion of the skull that is preserved, the snout length is estimated to be well more than half the length of the head. Among Acipenser, this is only otherwise seen in Acipenser stellatus, from the Black, Asov, and Caspian seas. If more complete specimens of †A. amnisinferos n. sp. are discovered and this phylogenetic affinity is supported, then this would indicate a biogeographic connection to the European sturgeon fauna.
Regardless of the phylogenetic positions of †Acipenser praeparatorum n. sp. and †A. amnisinferos n. sp., the presence of these two species, which are both close in morphological details to certain clades of extant sturgeons, points to the importance of the latest Cretaceous fossil record of freshwater and coastal members of the Acipenseridae for better understanding the evolution of this iconic family of fishes. New data on the skeletal anatomy of extant species, particularly for Ponto-Caspian and eastern Asian species, is necessary to conduct a robust phylogenetic study of the broad diversity of Late Cretaceous sturgeons. Although several modern river systems support a single species of Acipenseridae, many rivers have two or more species of sturgeons (e.g., six sympatric species in the Danube River system). Additional specimens discovered from the Tanis site in the future may yield additional information on the early phylogenetic, morphological, and ecological diversity of Acipenseridae.
Acknowledgments
We thank C. Van Beek and K. Passaglia for preparation of the FMNH specimens of the two species described herein, and J. Weinstein for photographic assistance. We thank A. Murray for providing some of the images used here. T. Larson and K. Mackenzie graciously provided access to the DMNH specimen examined; N. Nev-Yagle prepared the DMNH specimen. This study was supported by the US National Science Foundation (DEB-1754483) and grants from the Negaunee Foundation. EJH was also supported by a Bass Fellowship from the Field Museum that supported an extended visit to the Field Museum. We thank W.E. Bemis, A. López-Arbarello, and H.-D. Sues for helpful reviews and comments that improved the manuscript. This is contribution number 4111 of the Virginia Institute of Marine Science, William & Mary.






























