Introduction
Taxonomic research on early Paleozoic radiolarians from isolated occurrences is progressing rapidly compared to that for biostratigraphic correlation and paleoecological investigations, resulting in an improved understanding of systematics over the past two decades. Western Newfoundland, where more than a dozen radiolarian studies have been conducted during the last three decades, is one of the most intensively studied areas with numerous Lower and Middle Ordovician localities (Aitchison et al., Reference Aitchison, Flood and Malpas1998; Won and Iams, Reference Won and Iams2002, Reference Won and Iams2011, Reference Won and Iams2013, Reference Won and Iams2015a, Reference Won and Iamsb; Won et al., Reference Won, Iams and Reed2005, Reference Won, Iams and Reed2007; Maletz, Reference Maletz2007; Pouille et al., Reference Pouille, Danelian and Maletz2014; Kachovich and Aitchison, Reference Kachovich and Aitchison2020, Reference Kachovich and Aitchison2021). Similarly, the Guanzhuang section, Pingliang, China, is the most well-known locality for Upper Ordovician (Sandbian) radiolarians. It is stratigraphically well calibrated using abundant graptolites and conodonts in shale and limestone beds and was once a candidate for the Global Boundary Stratotype Section (GSSP) of the base of Upper Ordovician (Sun, Reference Sun1933; Chen et al., Reference Chen, Zhou, Li, Zou, Wang, Luo, Yao and She1984; Finney et al., Reference Finney, Bergström, Chen and Wang1999; Wang et al., Reference Wang, Bergström, Zhen, Chen and Zhang2013). Despite its importance, the Pingliang Formation has been explored only twice for radiolarians. The first investigation was nearly 30 years ago (Wang, Reference Wang1993), with the second two decades later (Wang et al., Reference Wang, Cheng and Zhang2010). This paper undertakes the first systematic investigation of radiolarian diversity of the Climacograptus bicornis Biozone in the Guanzhuang section. It discusses and resolves various long-held disputes regarding the status of several genera in the family Inaniguttidae with observations from models created using micro-computed tomography (micro-CT).
Samples examined in this study yielded a diverse, well-preserved assemblage with 19 radiolarian species representing 12 genera. Two new genera are introduced together with six new species and systematics are revised for all the taxa recovered. This diverse, well-preserved assemblage from the Guanzhuang section provides the first record of radiolarians from the late Sandbian (Sa2; Upper Ordovician) C. bicornis Graptolite Biozone. It bears similarity to both the collection of radiolarians reported by Wang (Reference Wang1993) from the same section and assemblages described from eastern Kazakhstan by Nazarov and Popov (Reference Nazarov and Popov1980).
Geological setting
Locality information
The Guanzhuang section is the type locality of the Pingliang Formation and located near Yindong, Guanzhuang, Kongtong District, ~8 km SW of Pingliang City, Gansu Province, China (35°29′32″N, 106°36′31″E, WGS 84; Fig. 1). This naturally exposed, ~90 m high, steep cliff is easily accessible and located alongside a small creek. Sedimentary rocks in the section are part of one of five sub-basins comprising the southwest Ordos Basin, which experienced several transitions in sedimentary facies throughout the Middle–Upper Ordovician (Chen et al., Reference Chen, Zhang, Wang, Goldman, Bergström, Fan, Finney, Chen, Chen, Bergström, Finney, Zhang, Fan, Chen, Goldman, Wang and Ma2017; Dai et al., Reference Dai, Qiang, Tian, Xi, Luo, Li, Liang, Wang and Li2019; Yang et al., Reference Yang, Loon, Jin, Jin, Han, Fan and Liu2019).
Figure 1. Geological map of the Pingliang region, Gansu Province, China (modified after Sun et al., Reference Sun, Yang, Dong, Yang, Peng and Zhao2020); inset map shows the location of the Ordos Basin.
Sediments at Guanzhuang were deposited in a carbonate platform-marginal slope environment, with deposition of Pingliang Formation interpreted to have occurred in a convergent-margin, back-arc setting in response to a series of tectonic events associated with the Qinling Orogeny (Wang et al., Reference Wang, Zhou, Wang, Zhang, Jing and Xing2015a, Reference Wang, Zhou, Wang, Jing and Zhangb; Yang et al., Reference Yang, Chen, Chen, Ding, Gao, Lei, Zhang, Shi and Tong2015; Zhang et al., Reference Zhang, Zhang, Zhao, Nie, Wang and Zhang2019). A few minor folds and faults can be observed, but the stratigraphy of the Ordovician succession is not significantly disrupted by tectonic activities.
Stratigraphic context
The Guanzhuang section is ~100 m thick. It overlies the Lower Ordovician Sandaogou Formation at its base and is unconformably overlain by the lower Permian Shanxi Formation (Fig. 2). It consists predominantly of limestone and shale with thin intercalations of sandstone, calcareous mudstone, and some debris flow deposits. A diverse assemblage of graptolites and conodonts extracted from shale and carbonate layers constrain the succession to the Sandbian, which is widely considered to represent the best example of the boundary between the Nemagraptus gracilis and C. bicornis biozones (Finney et al., Reference Finney, Bergström, Chen and Wang1999; Wang et al., Reference Wang, Bergström, Zhen, Chen and Zhang2013; Chen et al., Reference Chen, Zhang, Wang, Goldman, Bergström, Fan, Finney, Chen, Chen, Bergström, Finney, Zhang, Fan, Chen, Goldman, Wang and Ma2017).

Figure 2. Lithostratigraphy of the Pingliang Formation in the Guanzhuang section (modified after Wang et al., Reference Wang, Bergström, Zhen, Chen and Zhang2013) and correlation of biozones with co-occurring conodonts and graptolites. Scale bars are 100 μm where not specified.
Levels previously examined for radiolarians by Wang (Reference Wang1993) and Wang et al. (Reference Wang, Cheng and Zhang2010) are stratigraphically lower than those from which the two samples that produced the assemblage described herein were collected (see Fig. 2). Both previous studies on Pingliang radiolarians are from the N. gracilis Graptolite Biozone. Wang (Reference Wang1993) assigned a radiolarian fauna from bed 3 in the Pingliang section (after Chen et al., Reference Chen, Zhou, Li, Zou, Wang, Luo, Yao and She1984) to the Syndyograptus subzone of the N. gracilis Biozone. This 27.5 m thick unit begins 13 m above the base of the section. The conodont assemblage Pygodus anserinus, Periodan aculeatus Hadding, Reference Hadding1913, and Pygodus serra (Hadding, Reference Hadding1913) occurs in beds 2–8 (Wang, Reference Wang1993) representing the entirety of upper Darriwilian (Dw3) to lower Sandbian (Sa1). Co-occurring graptolites include N. gracilis and C. bicornis, which range through the Sandbian (Sa1 and Sa2) (Wang, Reference Wang1993). Although Chen et al. (Reference Chen, Zhou, Li, Zou, Wang, Luo, Yao and She1984) provided an account of graptolites and conodonts from bed 3, no age-specific taxa were included other than mentioning that bed 4 contains N. gracilis and P. anserinus. Later conodont and graptolite studies of the Guanzhuang section (Wang et al., Reference Wang, Bergström, Zhen, Chen and Zhang2013; Chen et al., Reference Chen, Zhang, Wang, Goldman, Bergström, Fan, Finney, Chen, Chen, Bergström, Finney, Zhang, Fan, Chen, Goldman, Wang and Ma2017; Dai et al., Reference Dai, Qiang, Tian, Xi, Luo, Li, Liang, Wang and Li2019) allow improved correlation of the radiolarian assemblage from bed 3, which can be assigned to the Sandbian (Sa1) N. gracilis Biozone. Wang et al. (Reference Wang, Cheng and Zhang2010) examined the basal section of the Guanzhuang succession and introduced the radiolarian genus Gansuceratoikiscum occurring within the Pygodus anserinus Conodont Biozone.
Materials and methods
Faunas described herein were extracted from micritic limestone samples collected from the Pingliang Formation in the Guanzhuang section. From 56 limestone samples, two samples yielded well-preserved radiolarians whereas most other samples only had poorly preserved, unrecognizable forms.
Samples were crushed into ~5 cm3 pieces to increase the reaction surface area prior to chemical digestion in order to release radiolarians from their surrounding matrix. Fragments were etched in 10% acetic acid for up to 12 weeks in the micropaleontology laboratory of the School of Earth and Environmental Sciences (SEES) of the University of Queensland. The residue produced through this slow etching process was later wet-sieved and the fraction between 500 μm and 63 μm was collected and air-dried. The procedure was repeated until a representative assemblage of radiolarians was recovered. Well-preserved, biostratigraphically significant radiolarians were individually picked under Leica M80 light microscope and later imaged using a desktop Hitachi TM3030 SEM.
Seven specimens with disputed and previously uncertain taxonomic affiliations were selected for detailed examination: Etymalbaillella toriterminalis Li, Reference Li1995; Haplentactinia baltica (Nazarov and Nolvak, Reference Nazarov and Nolvak1983), Haplotaeniatum implexa n. sp., Geminusphaera kongtongensis n. gen. n. sp., Inanigutta quadrispinosa n. sp., Kalimnasphaera pingliangensis n. sp., and Protopylentonema ordosensis n. gen. n. sp. All were scanned to produce two-dimensional tomographs using a Zeiss Xradia Versa XRM-500 high-resolution micro-CT scanner at the Julius Kruttschnitt Mineral Research Centre, The University of Queensland, following the method developed by Kachovich et al. (Reference Kachovich, Sheng and Aitchison2019). Avizo® 9.7 volume rendering software was used to generate 3D models of the specimens, which were subsequently used to facilitate taxonomic interpretations.
Repositories and institutional abbreviations
Specimens examined in this study are deposited in the micropaleontology collection of the School of Earth and Environmental Sciences, The University of Queensland, Australia (SEES-UQ). SEES/140703 refers to the collection number, while the middle two digits and the last three characters of the specimen identification number (SEES/140703-XX-YYY) correspond to the sample number and species identification number, respectively. Other repositories mentioned herein include Geological Institute, Moscow (GIN); Department of Geology, Tallinn University of Technology, Estonia (GIT); Paleontological Collection, Department of Geology of University of Alberta (UA); Institute of Geology, Beijing (BGIN); Nanjing Institute of Geology and Paleontology, Chinese Academy of Sciences (NIGPAS); and Australian Museum (AM.F).
Systematic paleontology
Phylum Radiozoa Cavalier-Smith, Reference Cavalier-Smith1987
Class Polycystina Ehrenberg, Reference Ehrenberg1838, sensu Riedel, Reference Riedel1967
Order Albaillellaria Deflandre, Reference Deflandre and Grassé1953, emend. Holdsworth, Reference Holdsworth1969
Remarks
The order Albaillellaria was introduced by Deflandre (Reference Deflandre and Grassé1953). Holdsworth (Reference Holdsworth1969b) revised the initial diagnosis to incorporate Ceratoikiscidae within the Albaillellaria. The order can be traced to the Ordovician with the earliest genus, Protoceratoikiscum Goto et al. (Reference Goto, Umeda and Ishiga1992). The basic skeletal framework of Albaillellaria is composed of three fundamental elements referred to as intersector, a-bar, and b-bar, which might differ in their length, position, or shape depending on the genus. These basic elements are clearly expressed in Albaillellidae and Ceratoikiscidae, while Follicucullidae display a reduced and simpler skeleton. Several authors have proposed a homologous relationship between Ceratoikiscidae and Albaillellidae that is explained via phylogenetic models reflecting the possibility of a common ancestor, possibly a primitive ceratoikiscid in the early Paleozoic (Foreman, Reference Foreman1963; Holdsworth, Reference Holdsworth1969a, Reference Holdsworthb; Won, Reference Won1983; Cheng, Reference Cheng1986; Renz, Reference Renz1988; Aitchison, Reference Aitchison1993). To accommodate all the genera from Ordovician to earliest Triassic that are presently assigned to this order, one should assume the initial skeleton of albaillellarians has undergone extensive modifications along the evolutionary pathway. De Wever et al. (Reference De Wever, Caulet, Nigrini and Caridroit2001) remarked, that during these skeletal alterations, albaillellarians were successful enough to always conserve at least one out of their three skeletal elements.
Family Ceratoikiscidae Holdsworth, Reference Holdsworth1969, sensu Holdsworth, Reference Holdsworth and Swain1977
Remarks
Initially this family included Ceratoikiscum and Holoeciscus, but has now been expanded to include more than a dozen genera spanning from Ordovician–Carboniferous. Foreman (Reference Foreman1963) introduced the basic nomenclature to describe the skeletal elements of ceratoikiscids, which was elaborated on later by several authors (Holdsworth, Reference Holdsworth1969b; Renz, Reference Renz1988; Noble and Lenz, Reference Noble and Lenz2007) (see Fig. 3.1, 3.2). Throughout their evolution, an architecture involving three principal rods that might have slightly reshaped or suppressed is retained as the most conspicuous feature of the Ceratoikiscidae, compared to highly variable development of patagial tissue and/or caveal ribs. Wakamatsu et al. (Reference Wakamatsu, Sugiyama and Furutani1990) stated that modifications among the caveal ribs are better regarded as features of lower levels of classifications. However, even the indisputable most primitive species, Protoceratoikiscum clarksoni Danelian and Floyd, Reference Danelian and Floyd2001, recovered from the Middle to Upper Ordovician chert beds in Southern Uplands, Scotland, displays a characteristic ‘triangular structure’ made from two primary spines and a straight by-spine that closely resembles the basic architecture of the three principal rods.
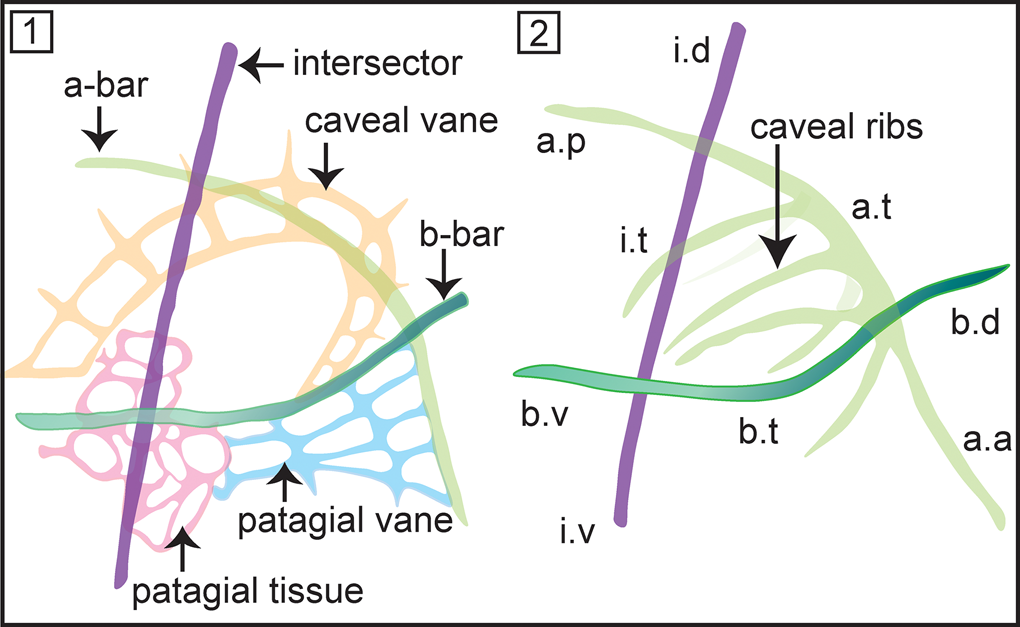
Figure 3. (1) Nomenclature describing the structural details of Ceratoikiscum. (2) Anatomical notation of the frame of Ceratoikiscum. i.d = intersector (extratriangular) dorsal portion; i.t = intersector triangle forming portion; i.v = intersector (extratriangular) ventral portion; a.p = a-rod (extratriangular) posterior portion; a.t = a-rod triangle forming portion; a.a = a-rod (extratriangular) anterior portion; b.d = b-rod (extratriangular) dorsal portion; b.v = b-rod (extratriangular) ventral portion; b.t = b-rod triangle forming portion (after Holdsworth, Reference Holdsworth1969b).
Genus Etymalbaillella Li, Reference Li1995
Type species
Etymalbaillella yennienii Li, Reference Li1995 (BGIN 930311) from the Baijingsi, Qilian Mountains, China, by original designation.
Diagnosis
Conical radiolarians with the patagium developed into a latticed framework enveloping the sub-triangular initial skeleton made of a prominent intersector along with a-bar and b-bar blended into the lattice. Bears an open anterior end and may have projections attached to the a-bar and intersector. Emended from Li (Reference Li1995).
Remarks
Etymalbaillella was first assigned to the Albaillellidae by Li (Reference Li1995). Constituent species include E. renzii, E. toriterminalis, and E. yennienii that were first reported from Baijingsi, Qilian Mountains, China (Li, Reference Li1995). Etymalbaillella was later grouped with Proventocitum and reassigned under the Proventocitiidae (Aitchison, Reference Aitchison1998). Noble and Webby (Reference Noble and Webby2009) recovered E. yennienii from the Malongulli Formation in Australia. To reflect the uncertainty about this assignment and to highlight the need for further investigation, it was placed under incertae sedis at both the Order and Family level by Noble et al. (Reference Noble, Aitchison, Danelian, Dumitrica, Maletz, Suzuki, Cuvelier, Caridroit and O'Dogherty2017). Known occurrences are significant because they come from deep marine (Li, Reference Li1995) and shallow marine (Noble and Webby, Reference Noble and Webby2009) facies. Both have yielded well-preserved shells in spite of their delicate nature. With reference to observations based on specimens observed in this study, the authors disagree with the comments of Noble and Webby (Reference Noble and Webby2009) on the lack of two lateral rods mentioned by Li (Reference Li1995) and the absence of albaillellid symmetry. In his discussion of the genus, Li (Reference Li1995, p. 338) noted the existence of “two rods along dorsal and ventral shell walls” united by “trabeculaea of circumferential ridge.” The nomenclature he applied reflects a preference towards the family Albaillellidae.
Considering the extensive discussions of phylogeny and homologies between Albaillellidae and Ceratokiscidae (Holdsworth, Reference Holdsworth1969a, Reference Holdsworth1971; Won, Reference Won1983; Cheng, Reference Cheng1986; Renz, Reference Renz1988; Aitchison, Reference Aitchison1993), both the aforementioned families undeniably share an architecture involving three principal rods, more or less modified depending on the context. The skeletal architecture that separates Ceratokiscidae and Albaillellidae is subtle, such that even a slight modification to the configuration can prompt confusion, which easily could make the case for a primitive morphotype. Therefore, emphasis should be placed on the stratigraphic continuity of forms when skeletal architecture is not obvious enough to proceed with a solid phylogenetic assignment. Cheng (Reference Cheng1986) remarked that acquisition of a shell, offset trabeculae on two rods, and wings, developed at a much later evolutionary stage. This explains the first occurrence of Albaillellidae in the Late Devonian (Cheng, Reference Cheng1986), while ceratokiscids made their entrance by the Middle Ordovician (Danelian and Floyd, Reference Danelian and Floyd2001). In spite of superficial resemblance of Etymalbaillella to Albaillella, there is little evidence to fill the stratigraphic gap between the first known occurrences of Albaillella and Etymalbaillella. This indicates the possibility of assigning Etymalbaillella to the Ceratoikiscidae because it also shares the same three-rod architecture and is compatible with the period of origin. Nevertheless, the possibility of finding a morphotype intermediate between Albaillella and Etymalbaillella in the stratigraphic gap cannot be completely eliminated, in which case further family-level revision may be required.
Etymalbaillella toriterminalis Li, Reference Li1995
Figures 4, 11.5
- Reference Li1995
Etymalbaillella toriterminalis Li, p. 338, pl. 2, figs. 12, 16.
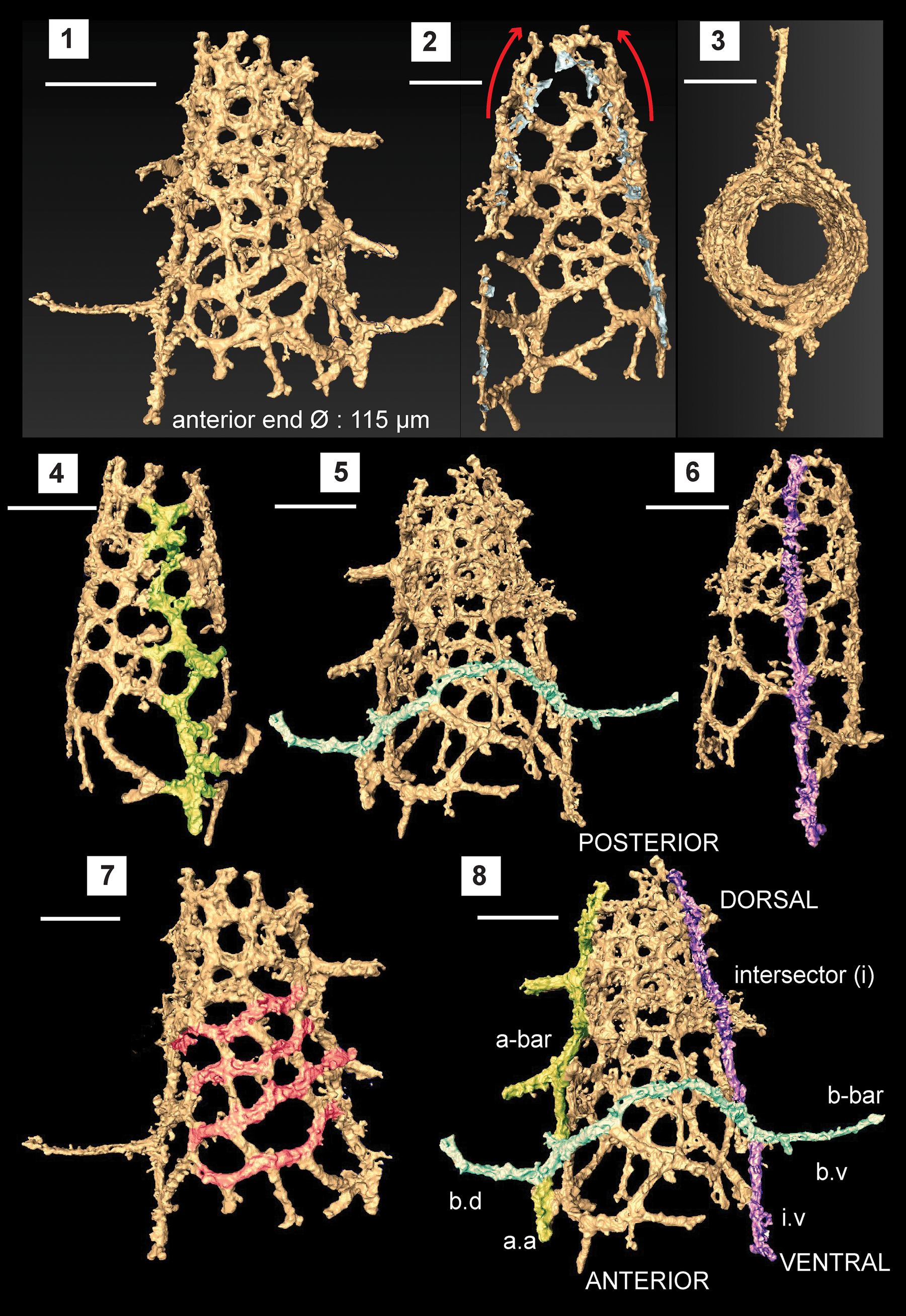
Figure 4. Structural details highlighted using a micro-CT model of Etymalbaillella toriterminalis (SEES/140703-49-ET2). (1) Complete specimen of E. toriterminalis; (2) inflated top area of the shell; (3) view from the anterior end towards the apex highlighting the skeleton symmetry; (4) a-bar blended into the lattice shell (Ortho view); (5) b-bar blended into the lattice shell (Ortho view); (6) intersector (Ortho view); (7) diagonal configuration of interpore areas, possibly indicating traces of caveal ribs; (8) anatomical notation of frame with ventral side facing front; a.a = a-bar (anterior portion); b.d = b-bar (dorsal portion) b.v = b-bar (ventral portion); i.v = intersector (ventral portion). Scale bars = 100 μm.
Holotype
Neotype specimen no. SEES/140703-55-ET1 (Fig. 11.5) and SEES/140703-49-ET2 (digitized as Fig. 4 and Supplemental Data 1) from Sandbian limestone beds in the Pingliang Formation, Guanzhuang section, Gansu Province, China.
Diagnosis
Conical skeleton composed of a latticed framework supported by and merged into the three-rod ceratoikiscid architecture; intersector, a-bar, and b-bar. B-bar extends laterally forming extratriangular segments at ventral and anterior positions. Slightly inflated close to the point of convergence between a-bar and intersector. Emended from Li (Reference Li1995).
Description
Observations using micro-CT reveals important skeletal features that suggest possible phylogenetic affiliation to the Ceratoikiscidae or Albaillellidae. Careful segmentation of the E. toriterminalis skeleton reveals the presence of an almost straight intersector (Fig. 4.6) along with an a-bar (Fig. 4.4) and b-bar (Fig. 4.5) blended with the latticed shell that forms the fundamental initial skeleton in ceratoikiscids and albaillellids. Intersections of the intersector with the b-bar and the a-bar with the b-bar produce prominent extratriangular segments at ventral and anterior junctions, respectively (Fig. 4.8). Extensions of the b-bar beyond the shell frame have the appearance of a pair of wings and show a maximum length of 80 μm. A-bar shows a slightly offset branching and makes up the lattice frame along with 1–2 wing-like extensions that project outwards from the shell. These wing-like extensions from the a-bar are much shorter than dorsal and ventral portions of the b-bar (Fig. 4.8). The shell is slightly inflated close to the point of convergence between the a-bar and intersector, which could be a result of the tendency of the a-bars to curve when present in typical ceratoikiscid frames (Fig. 4.2). There are no conspicuous caveal ribs, but it may be assumed that they have been reduced in forming the lattice frame in conjunction with the patagium. The continuous diagonal inter-pore areas highlighted in Figure 4.7 also can be interpreted as residual traces of caveal ribs.
Pores in the lattice are sub-rounded to polygonal and are arranged diagonally in rows. These pores gradually reduce in size towards the posterior end of the intersector. Lattice meshwork may be considered a type of primitive equivalent to the patagium seen in Ceratoikiscidae, although patagial vanes or tissue cannot be resolved specifically. The vertical length of the entire conical shell made of the latticed framework is 225 μm, excluding the possibly broken apex. The widely open anterior end is 115 μm in diameter (Ø), but is less than 65 μm at the posterior end. All specimens encountered have open posterior ends, although they can be interpreted as broken ends possibly as a result of taphonomic conditions.
Remarks
This is the first report of E. toriterminalis after the initial discovery from Baijingsi, Qilian Mountains in China. The original type material (BGIN 930851) has been destroyed (Li Hongsheng personal communication, 2020, via Luo Hui), so a neotype (SEES/140703-55-ET1) is designated herein. Specimens are extremely delicate and frequently leave behind molds upon fragmentation. Use of the terms adopted herein to describe the structural details of the species follows the terminology and the definitions revised by Holdsworth (Reference Holdsworth1969b). For E. toriterminalis, the presence of ventral and anterior extratriangular segments indicates a bias towards Ceratoikiscidae, even if the aspect of stratigraphic continuation is overlooked. Etymalbaillella toriterminalis is relatively small in dimensions when compared to E. renzii. It is distinguished from E. yennienii by having longer maximum lengths for wings.
Etymalbaillella renzii Li, Reference Li1995
Figure 11.10
- Reference Li1995
Etymalbaillella renzii Li, p. 338, pl. 1, figs. 1, 3, 5.
Holotype
Neotype specimen no. SEES/140703-49-ER1 (Fig. 11.10) from Sandbian limestone beds in the Pingliang Formation, Guanzhuang section, Gansu Province, China.
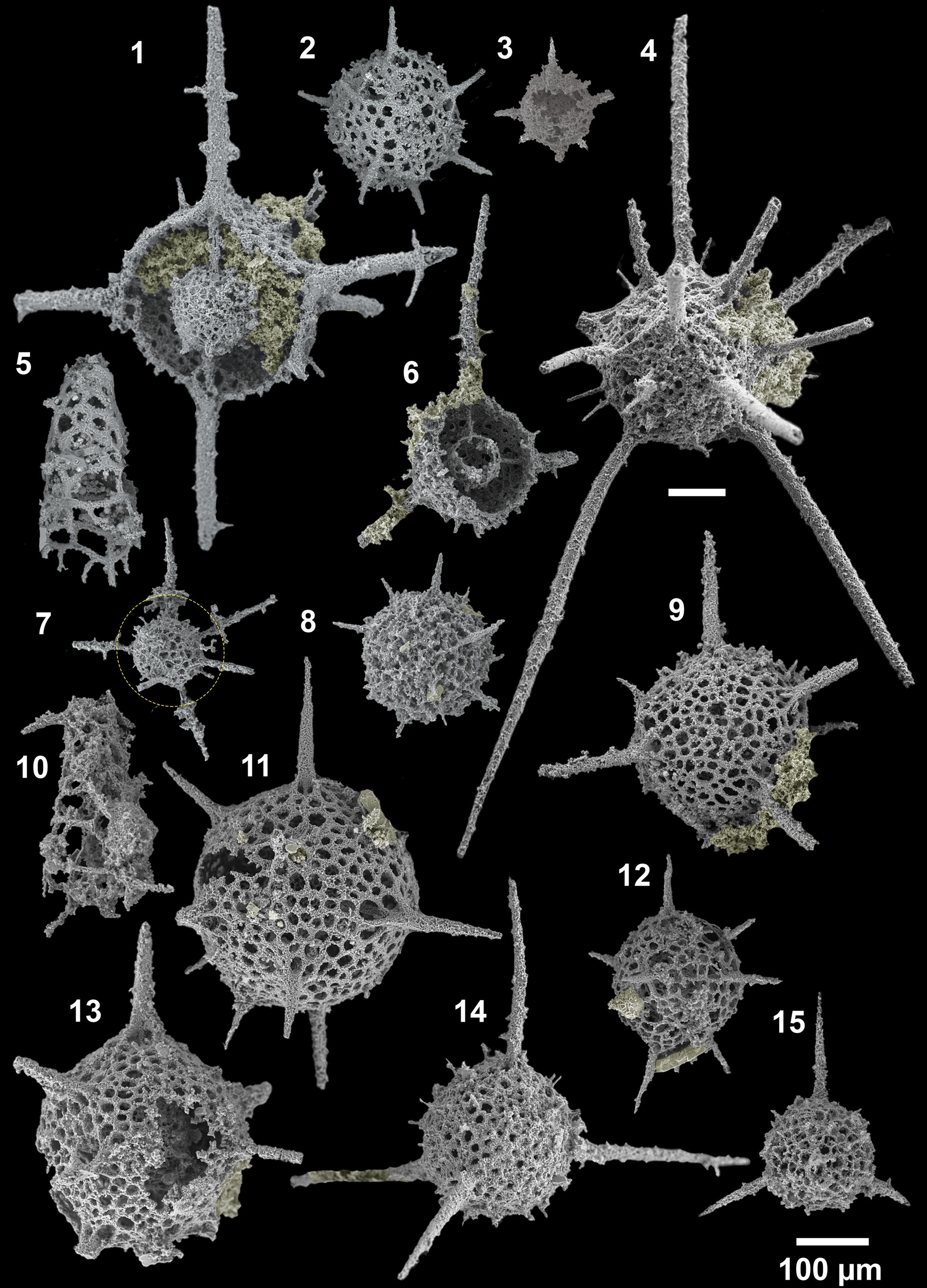
Figure 11. SEM images of radiolarians extracted from bed 5 of the Pingliang Formation, Guanzhuang section, Gansu, China. (1) Inanibigutta pinglianensis Wang, SEES/140703-55-IP1; (2) Oriundogutta bella Wang, SEES/140703-55-OB1; (3) ?Oriundogutta miscella minuta Wang, SEES/140703-55-OM1; (4) Kalimnasphaera pingliangensis n. sp., holotype, SEES/140703-55-KP1; (5) Etymalbaillella toriterminalis Li, neotype, SEES/140703-55-ET1; (6) Inanibigutta verrucula (Nazarov and Popov), SEES/140703-49-IV1; (7, 12) Geminusphaera kongtongensis n. gen. n. sp., (7) SEES/140703-55-GK3, (12) holotype, SEES/140703-55-GK1; (8) Haplotaeniatum implexa n. sp., holotype, SEES/140703-55-HI1; (9) Inanigutta complanata (Nazarov), SEES/140703-55-IC1; (10) Etymalbaillella renzii Li, neotype, SEES/140703-49-ER1; (11) Geminusphaera grandis n. gen. n. sp., holotype, SEES/140703-55-GG1; (13) Protopylentonema ordosensis n. sp., SEES/140703-49-PO1; (14) Inanigutta gansuensis Wang, SEES/140703-55-IG1; (15) Inanigutta quadrispinosa n. sp., holotype, SEES/140703-49-IQ1. Scale bar is 100 μm.
Diagnosis
Conical skeleton composed of a latticed framework supported by and merged into the three-rod ceratoikiscid architecture; intersector, a-bar, and b-bar. Downward-facing pair of wings extends from the upper section of the intersector and a-bar. Emended from Li (Reference Li1995).
Description
Conical lattice frame is at least 300 μm long, excluding the broken apex of the specimens. The anterior end is wide open with Ø = 112 μm. The apical section of the shell develops a pair of identical wing-like projections, facing downward, having an approximate length of 50 μm. The rest of the shell is devoid of wings, unlike E. toriterminalis and E. yennienii. Pores are polygonal and gradually reduce in size upon reaching the posterior section of the shell. Pore sizes at the anterior end are larger than those seen in other species of Etymalbaillella.
Remarks
A single specimen was recovered from the Pingliang Formation in the Guanzhuang section. The original type material (BGIN 930919) has been destroyed (Li Hongsheng personal communication, 2020, via Luo Hui), so a neotype (SEES/140703-49-ER1) is designated herein. The specimen is moderately well preserved and SEM observations are not of sufficient clarity to allow commenting on the initial skeleton configuration. Nevertheless, the presence of identical apical protrusions and a conical lattice frame allows confident identification. The emended diagnosis of the species is based on the initial skeletal details revealed through the micro-CT model constructed for E. toriterminalis and the SEM observations of E. renzii.
Genus Gansuceratoikiscum Wang, Cheng, and Zhang, Reference Wang, Cheng and Zhang2010
Type species
Gansuceratoikiscum guanzhuangensis Wang et al., Reference Wang, Cheng and Zhang2010 (pl. 1, fig. 11) from Pingliang Formation in the Guanzhuang section of the Gansu Province, China by original designation.
Gansuceratoikiscum guanzhuangensis Wang, Cheng, and Zhang, Reference Wang, Cheng and Zhang2010
Figure 12.1–12.5
- Reference Wang1991
Ceratoikiscum sp. 1; Wang, p. 249, pl. 1, figs. 6, 7.
- Reference Wang, Cheng and Zhang2010
Gansuceratoikiscum guanzhuangensis Wang et al., p. 475, pl. 1, figs. 1–30.
Holotype
Specimen in Wang et al. (Reference Wang, Cheng and Zhang2010, pl. 1, fig. 11) from limestone beds in the Pingliang section, Gansu Province, China.
Description
Specimen 1 in Figure 12 appears to have the patagial tissue, a-bar, and b-bar, while specimens in Figure 12.3 and 12.4 display different numbers of caveal ribs originating from the a-bar. The specimen in Figure 12.2 could be a juvenile form with all three rods, a row of arch, and a median bar (sensu Wang et al., Reference Wang, Cheng and Zhang2010).
Remarks
Specimens recovered are most likely to be fragments of G. guanzhuangensis. Proholoeciscus foremanii reported from Qilian Mountains, China (Li, Reference Li1995), which is now regarded as a nomina dubia (Noble et al., Reference Noble, Aitchison, Danelian, Dumitrica, Maletz, Suzuki, Cuvelier, Caridroit and O'Dogherty2017), may also be a fragment of G. guanzhuangensis because it appears similar to the specimen in Figure 12.3.
Order Entactinaria Kozur and Mostler, Reference Kozur and Mostler1982
Family Haplentactiniidae Nazarov in Nazarov and Popov, Reference Nazarov and Popov1980 (nomen translatum Nazarov and Ormiston, Reference Nazarov and Ormiston1984)
Genus Bissylentactinia Nazarov, Reference Nazarov1975
Type species
Bissylentactinia rudicula Nazarov, Reference Nazarov1975 (GIN. No. 4046-19) from the Egindinskaya series, Southern Urals, Northern Mugodzhars, Aitpaika River by original designation.
Bissylentactinia bifida Nazarov and Popov, Reference Nazarov and Popov1980
Figure 12.11
- Reference Nazarov and Popov1980
Bissylentactinia bifida Nazarov in Nazarov and Popov, p. 64, pl. XV, fig. 2.
Holotype
Specimen (GIN 4333/22) from the Middle Ordovician, Bestamaksk Formation, SW foothills of Chingiz Range, eastern Kazakhstan (Nazarov and Popov, Reference Nazarov and Popov1980, pl. XV, fig. 2).
Remarks
The specimen encountered clearly demonstrates ‘grouped branching’ developing at a certain distance from the median point, which distinguishes this genus from other members of the Palaeoscenidiidae. Because the studied assemblage includes several fragments of members belonging to the Palaeoscenidiidae, identification of Bissylentactinia would be challenging, if not for the grouped apophyses. The type specimen of B. bifida illustrated by Nazarov and Popov (Reference Nazarov and Popov1980) differs from the material in this study in having slightly longer (224 μm) spines. The specimen illustrated does not fully capture the length of the two hetero-polar spines. Apophyses on two spines are also broken. This is the first report of B. bifida from the Ordovician since erection of this species by Nazarov and Popov (Reference Nazarov and Popov1980). The long stratigraphic gap existing between Darriwilian (Nazarov and Popov, Reference Nazarov and Popov1980) and Frasnian (Nazarov, Reference Nazarov1975) occurrences can now be reconciled with the intervening occurrence in the Sandbian reported herein.
Genus Haplentactinia Foreman, Reference Foreman1963
Type species
Haplentactinia rhinophyusa Foreman, Reference Foreman1963 (USNM 640392) from the Norwalk 1 calcareous concretions of the Upper Devonian Huron Member of the Ohio Shale by original designation.
Haplentactinia baltica Nazarov in Nazarov and Nolvak, Reference Nazarov and Nolvak1983
Figure 5, Supplemental Data 2
- Reference Nazarov and Popov1980
Haplentactinia sp. Nazarov in Nazarov and Popov, p. 55, pl. XVII, fig. 4.
- Reference Nazarov and Nolvak1983
Haplentactinia baltica Nazarov in Nazarov and Nolvak, p. 5, pl. II, figs. 2–4.
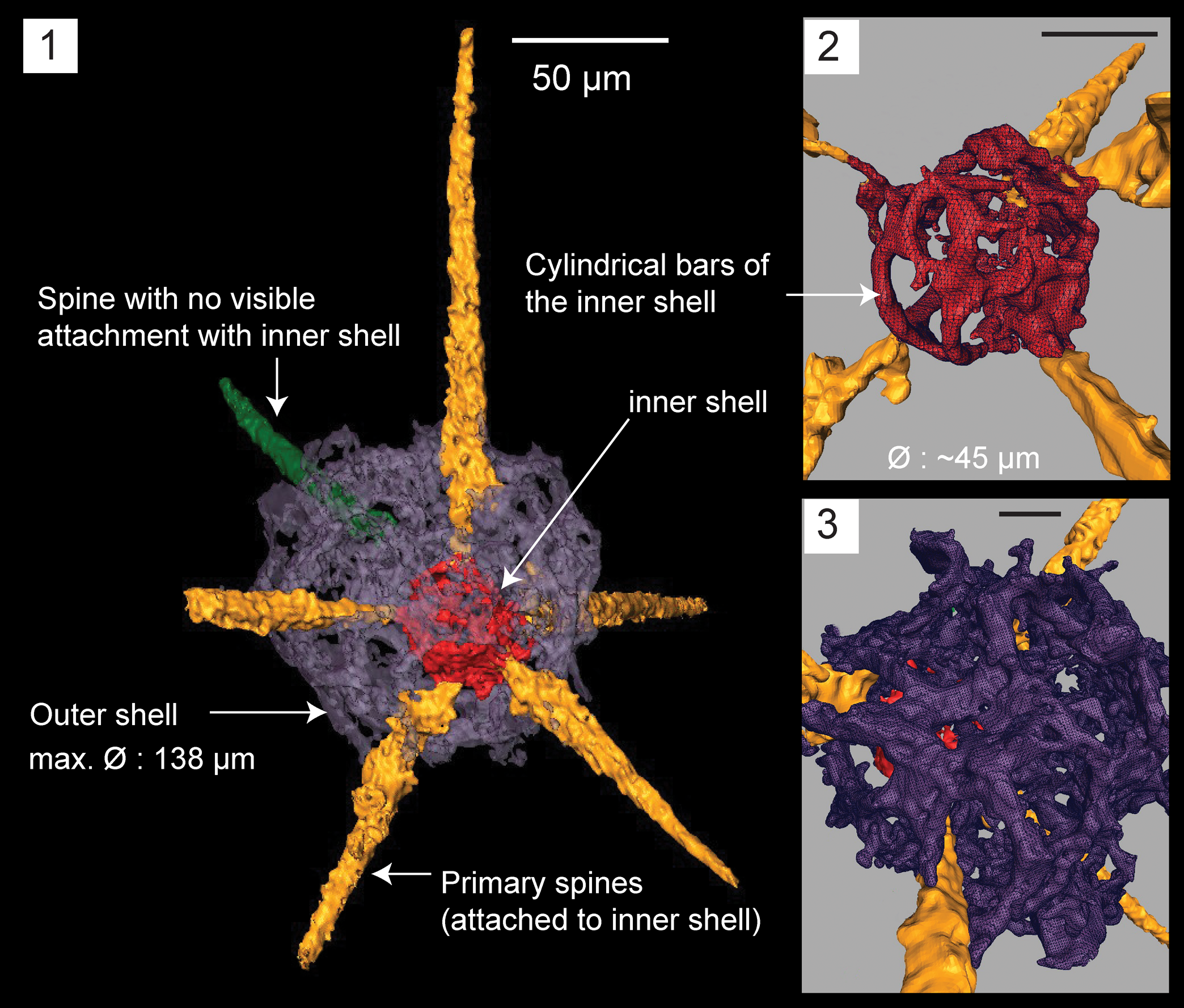
Figure 5. Structural details highlighted using a micro-CT model for Haplentactinia baltica (SEES/140703-55-HB1). (1) Complete specimen; (2) inner shell with five spines attached; (3) lattice meshwork of the outer shell. Scale bars (1) 50 μm; (2, 3) 25 μm.
Holotype
Specimen (GIT 182-4), former no. GIN R2/5408, from the Upper Ordovician, Saunja Formation in Estonia (Nazarov and Nolvak, Reference Nazarov and Nolvak1983, pl. II, figs. 2–4).
Description
Micro-CT observations on specimen SEES/140703-55-HB1 reveal a clearly discernible outer shell, eccentrically positioned inner shell, and six primary spines that originate from the latter (Fig. 5.1). The point of origination of one of the spines cannot be clearly determined. The outer shell is polygonal and made of an irregular lattice developing from apophyses of the primary spines. Apophyses develop at variable distances from the inner shell and are interconnected laterally and obliquely, producing an intertwined mesh. Because of these interconnections, wide sub-circular and polygonal-shaped spaces (Ø ≈ 20 μm) develop on the outer shell (Fig. 5.3). The maximum outer shell Ø ≈ 138 μm. The inner shell is made of delicate ‘bandages’ (sensu Won and Iams, Reference Won and Iams2011) and cylindrical strands of varying thicknesses coalescing to form a spheroid with a maximum Ø = 45 μm (Fig. 5.2). The lengths of gently tapering spines vary between 80–140 μm. Slightly curved or straight spines are both present.
Remarks
The inner spicule mentioned in the species description of Nazarov and Nolvak (Reference Nazarov and Nolvak1983) was not observable in our scanned specimen. However, the inner shell is composed of thin cylindrical strands, which might be easily confused with a spicule. Only one specimen was found and the fair probability of the inner spicule not being preserved in it prevents further revision to the systematics of the species. The first encounter of this species is from the Ulkuntas Member, which is Hirnantian in age (Nazarov and Popov, Reference Nazarov and Popov1980; Aitchison et al., Reference Aitchison, Suzuki and O'Dogherty2017a). The Saunja Formation of Estonia where the type specimen was introduced is of Nabala Stage and coincides with the uppermost Sandbian. Although H. baltica was apparently a rare species in the Late Ordovician, the distribution in three isolated paleo-plates (Baltica, N. China, and Kazakh) terranes accounts for its successful dispersal.
Family Palaeoscenidiidae Riedel, Reference Riedel1967
Genus Palaeoephippium Goodbody, Reference Goodbody1986, sensu MacDonald, Reference MacDonald2004
Type species
Palaeoephippium bifurcum (UA 7155) from the Cape Phillips Formation type section, Cornwallis Island, N.W.T, Canada (Goodbody, Reference Goodbody1986, pl. 4, figs. 1–4).
Palaeoephippium radices Goodbody, Reference Goodbody1986, sensu MacDonald, Reference MacDonald2004
Figure 12.6, 12.9, 12.10
- Reference Goodbody1986
Palaeoephippium radices Goodbody, p. 144, pl. 4, figs. 9–12.
- Reference Li1994
Palaeoephippium radices; Li, p. 266, pl. 2, fig. 6.
- Reference Amon, Braun and Ivanov1995
Palaeoephippium radices; Amon et al., p. 6, text fig. 7.
- Reference MacDonald2004
Palaeoephippium radices; MacDonald, p. 271, figs 5.1–5.4, 8.1–8.4.
Holotype
Specimen (UA 7166) from the Cape Phillips Formation, Cornwallis Island, N.W.T, Canada (Goodbody, Reference Goodbody1986, p. 144, pl. 4, figs. 9, 10).
Description
Six rays, three from each end of a median bar. One apical ray, one intermediate ray, and one basal ray positioned at each end, bifurcating at short distances from the median bar. All six rays terminate with sharp ends.
Remarks
One nearly complete specimen (Fig. 12.10) was encountered, together with several bifurcated fragments. Although Li (Reference Li1995) mentioned this genus on an occurrence chart, no figures or descriptions were provided.
Palaeoephippium spp.
Figure 12.7, 12.8
Remarks
Species level identification is not possible because the specimens are highly fragmentary. However, spines with point- or bar-centered apical, intermediate or basal distribution, and subsequent bifurcation are most likely to be associated with Palaeoephippium. There is a close resemblance between the specimen (Fig. 12.8) and P. tricorne Goodbody illustrated in Goodbody (Reference Goodbody1986, fig. 6.1), which illustrates a fragment, indicating Figure 12.8 could also be P. tricorne. The specimen in Figure 12.7 has six rays, one of which is apical. Three short rays occupy an intermediate position, and the remaining two basal rays bifurcate at least twice before terminating with pointed ends. The intersection of these six rays is at a point rather than a median bar.
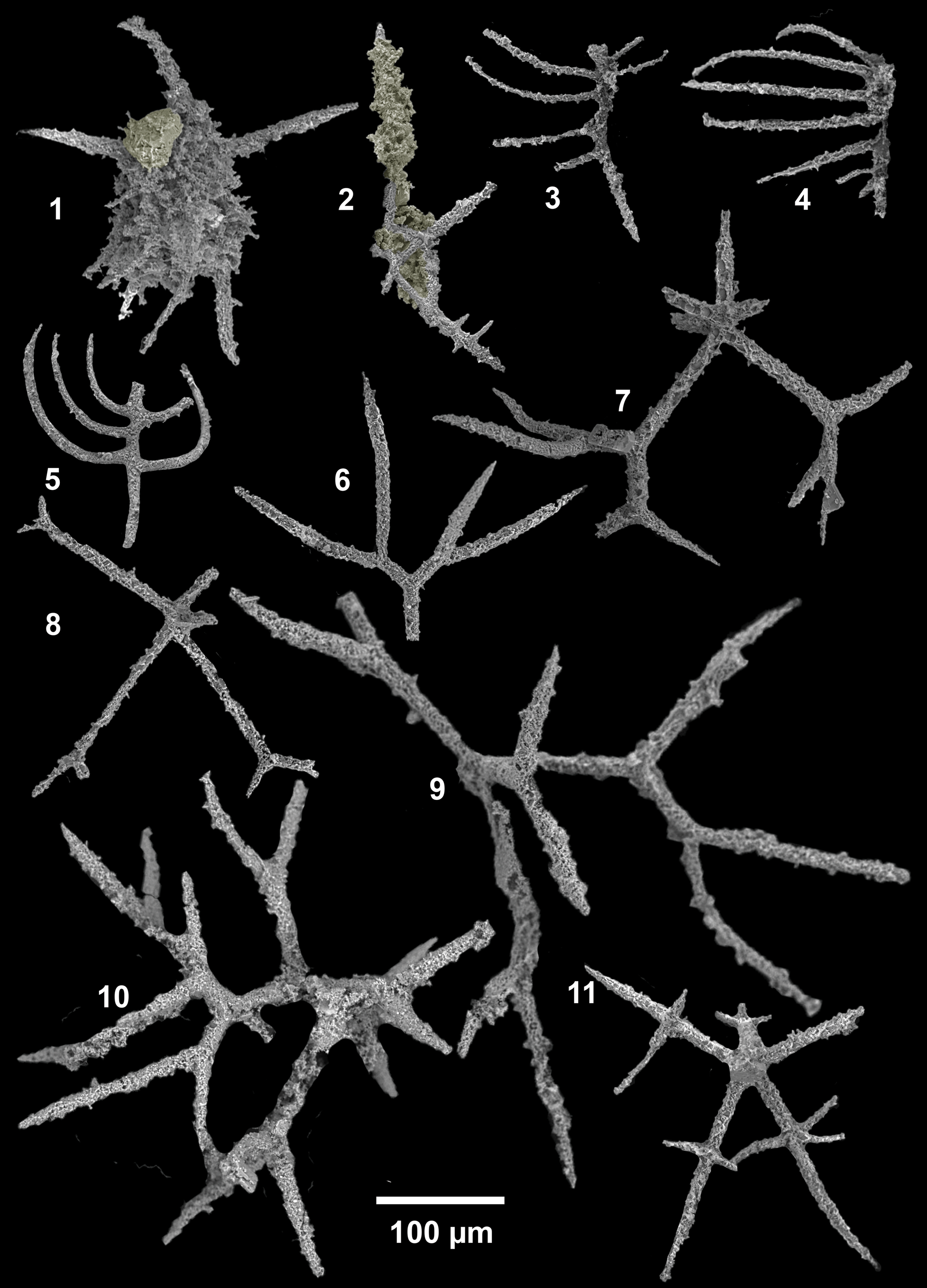
Figure 12. SEM images of radiolarians extracted from bed 5 of the Pingliang Formation in the Guanzhuang section, Gansu, China. (1–5) Gansuceratoikiscum guanzhuangensis SEES/140703-49-GG1–5; (6, 9, 10) Palaeoephippium radices Goodbody, (6) bifurcating fragments, SEES/140703-55-PR1, (9, 10) SEES/140703-49-PR1–2; (7, 8) Palaeoephippium spp., SEES/140703-55-PSP1–2; (11) Bissylentactinia bifida Nazarov and Popov, SEES/140703-55-BB1. Scale bar is 100 μm.
Family Pylentonemidae Deflandre, Reference Deflandre1963
Genus Protopylentonema new genus
Type species
Protopylentonema ordosensis n. gen. n. sp. (SEES/140703-49-PO1) from the Pingliang Formation in the Guanzhuang section,, Gansu Province, China.
Diagnosis
Pylomate radiolarian with an initial skeleton in the form of five rays arranged in two nearly perpendicular planes. One regularly porous sub-spherical shell envelopes the rays. Rays may develop apophyses midway that coalesce to form a second perforated shell. A few thin beams originate from the second shell to penetrate the cortical shell as short or long conical spines. Simple circular pylome is positioned near one or two of the primary spines. Primary and secondary spines often develop buttresses on cortical shell. Few to numerous conical by-spines.
Occurrence
Darriwilian to Sandbian from Kazakhstan and China (Nazarov, Reference Nazarov1975; Nazarov et al., Reference Nazarov, Popov and Apollonov1975; Nazarov and Popov, Reference Nazarov and Popov1980; Wang, Reference Wang1993; herein).
Etymology
In the sense of ‘Primitive Pylentonema’
Remarks
Protopylentonema n. gen. is introduced in this study to include Ordovician pylomate forms possessing a distinctive spicular system that represent entactinarians. Constituent species include Protopylentonema insueta (Nazarov, Reference Nazarov1975), Protopylentonema aperta (Nazarov et al., Reference Nazarov, Popov and Apollonov1975), Protopylentonema rimata, (Nazarov and Popov, Reference Nazarov and Popov1980), and Protopylentonema ordosensis n. sp.. The original species descriptions for P. aperta, P. rimata, and P. insueta clearly state the presence of centrally positioned, nearly perpendicularly arranged five spines and in the case of P. rimata and P. insueta, the existence of a vague inner shell developed from apophyses (Nazarov, Reference Nazarov1975; Nazarov and Popov, Reference Nazarov and Popov1980). These features accord with the micro-CT model created for P. ordosensis; the type species of the new genus. Nazarov and Ormiston (Reference Nazarov and Ormiston1993) reassigned these species (P. aperta, P. rimata, and P. insueta) to the genus Cessipylorum. Subsequently, Noble and Webby (Reference Noble and Webby2009) revised the genus Cessipylorum and synonymized it with Kalimnasphaera. Micro-CT comparison of Protopylentonema ordosensis n. sp. and Kalimnasphaera pingliangensis n. sp. demonstrates this assignment is not appropriate and that the taxa described earlier by Nazarov (Reference Nazarov1975) and Nazarov and Popov (Reference Nazarov and Popov1980) are best placed within the new genus Protopylentonema.
Protopylentonema ordosensis new species
Figures 6, 11.13
- Reference Wang1993
Inanihella penrosei Wang, pl. 3, fig. 1.
- Reference Wang1993
Cessipylorum cf. aperta Wang, pl. 4, figs. 7, 8.
Holotype
Specimen no. SEES/140703-49-PO1 (Fig. 11.13) and paratype SEES/140703-49-PO2 (digitized as Fig. 6 and Supplementary Data 3) in the micropaleontology collection of SEES-UQ. Known from Sandbian limestone beds in the Pingliang Formation, Guanzhuang section, Gansu Province, China.
Diagnosis
Pylentonemid radiolarian consisting of two sub-spherical shells and a five-rayed initial spicule; two bi-polar rays and three rays lying in a nearly perpendicular plane. All five rays pass through the two shells to form long tapering primary spines with buttresses on outer shell. One bi-polar ray, emerging as a primary spine, guards the circular pylome. Three secondary spines initiate at the inner sphere and form buttresses upon penetrating the outer shell. Few by-spines originate at the surface of the outer shell.
Occurrence
Sandbian from China (Wang, Reference Wang1993, and herein).
Description
Skeleton consists of two sub-spherical shells. Outer shell (Ø = 285 μm) comprises a regularly porous network with circular pores (Ø, 5–15 μm) (Fig. 6.5). Pore frames maintain a consistent thickness (5 μm), except at junctions and in the vicinity of spines. The initial skeleton consists of five rays that lie upon two nearly perpendicular planes. Two of the five rays are bi-polar and positioned on one plane. Three of the five rays are arranged on a plane nearly perpendicular to the other (Fig. 6.8). The micro-CT scan did not reveal the exact structure of the meeting point (sensu Won and Below, Reference Won and Below1999) because secondary infillings occupied the site. An element rendered partially transparent that is illustrated in Figure 6.8 shows a polyhedron floating at the middle of five rays. When the rays are projected inwards, two sets of rays create separate articulation points, suggesting the possibility of the presence of an intervening bar (Fig. 6.3). This feature is short and fits the definition of a micro-bar (sensu Won and Below, Reference Won and Below1999). Approximately 65 μm from the meeting point, rays develop apophyses that coalesce to form the delicate inner shell Ø = 125 μm (Fig. 6.1). The shell is sub-spherical and unevenly perforated with polygonal rims developed around pores of varying sizes. Rays attain a wider Ø before they grow beyond the inner shell. They convert to beams (Ø = 15 μm) and rise to the surface of the outer shell. A set of three thinner beams (Ø = 7–8 μm) initiates from pore-frame junctions of the inner shell (Fig. 6.1). These secondary beams also penetrate the surface of the external shell. Primary and secondary beams that convert to spines branch trichotomously or quadrachotomously just before interception with the outer shell (Fig. 6.7) and develop massive buttresses on the surface after penetration (Fig. 6.6). This imposes a slightly hexagonal shape to the outer shell as the six massive buttresses develop on the sphere (Fig. 6.5). The primary and secondary spines cannot be differentiated after surfacing and develop into distally tapering spines with lengths subequal to or slightly less than the shell Ø. Average Ø ≈ 45 μm near the base of a spine. The pylome (Ø = 55 μm) is generally sub-circular and has no rim (Fig. 6.2). It develops near one of the bi-polar rays that grows into a main spine. Since the pylome is commonly regarded as the posterior end of protozoans, the opposing main spine can be referred to as an apical spine (see Fig. 6.4). Very few short (35 μm), conical by-spines occur on pore frame junctions of the outer shell.
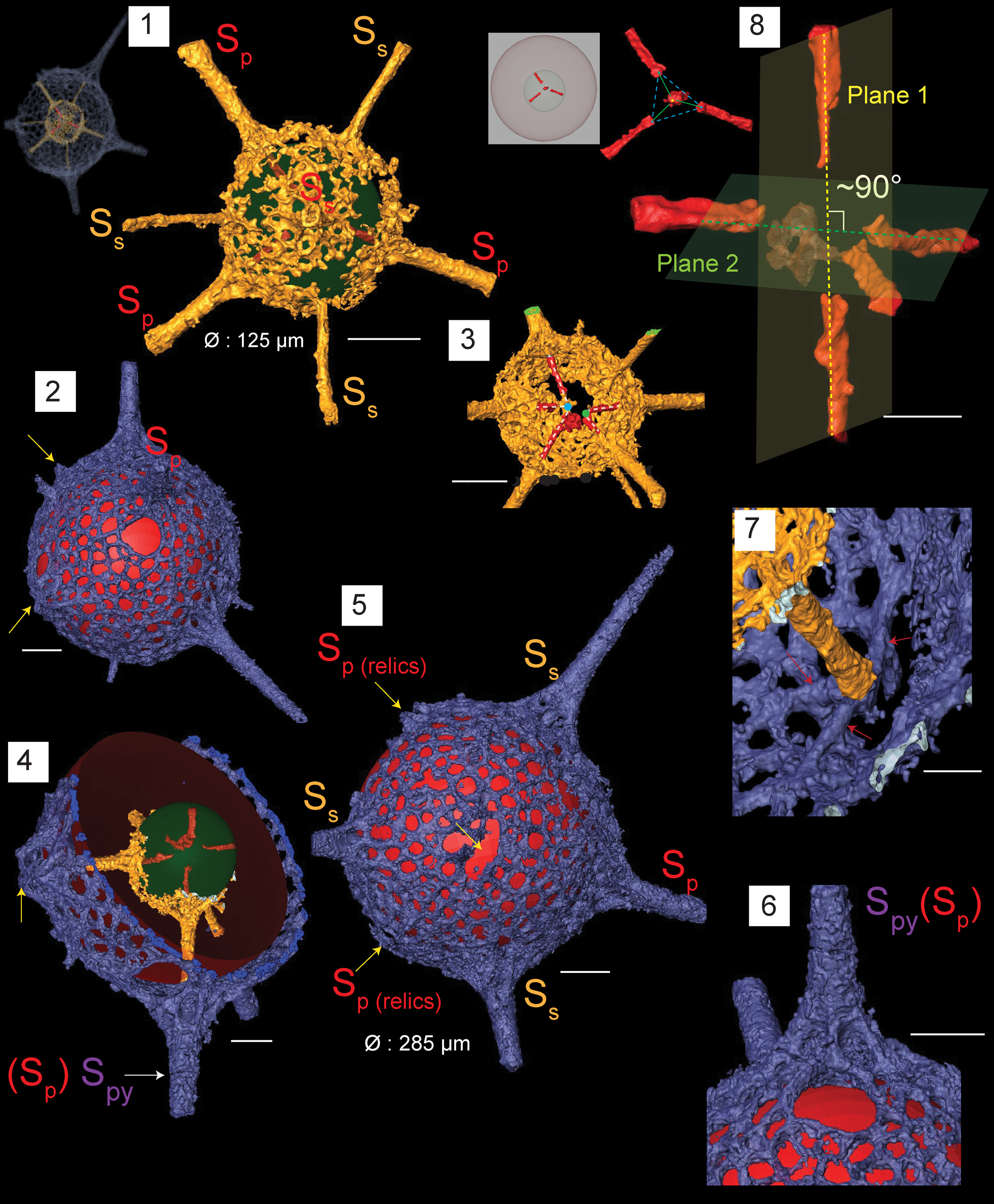
Figure 6. Structural details highlighted using micro-CT model for Protopylentonema ordosensis n. sp. (paratype, SEES/140703-49-PO2). SP: primary spines initiating from initial spicule. SS: secondary spines initiating from inner sphere. SPy: spines associated with pylome. Red and green spheres are inserted to aid visualization; yellow arrows indicate relics of SP. (1) Inner sphere and distribution of primary spines (SP) and secondary spines (SS) relative to the initial skeleton; (2) structure of pylome; (3) five rays displaying two separate meeting points; (4) position of pylome; (5) surface orientation of primary and secondary spines imposing a hexagonal appearance to the shell; (6) buttressed primary spine located near pylome; (7) branching of spines at the intersection with outer shell; red arrows indicate quadrachotomous branching possibly with one branch broken; (8) initial skeleton showing five-rayed spicule arranged along two nearly perpendicular planes. Scale bars are (1–6) 50 μm; (7, 8) 25 μm.
Etymology
Named after the Ordos Basin, in which sediments of the Pingliang Formation were deposited.
Remarks
A micro-CT model demonstrates the role of the initial skeleton in creating the two shells. Because the model does not reveal the structure of the meeting point of rays, there is a possibility of having a point-centered, five-rayed spicule or a polyhedron (sensu Nazarov and Popov, Reference Nazarov and Popov1980) instead of the micro-bar. Nazarov et al. (Reference Nazarov, Popov and Apollonov1975) preferred the words ‘polyhedron’ and ‘hexahedron’ to describe the center structure of P. aperta and P. insueta. The number of secondary spines that develop on the inner or external shell along with shell dimensions can be used to differentiate between species of this genus, while five primary spines will be a constant.
Order Spumellaria Ehrenberg, Reference Ehrenberg1876
Family Haplotaeniatidae Won, Blodgett, and Nestor, Reference Won, Blodgett and Nestor2002
Genus Haplotaeniatum Nazarov and Ormiston, Reference Nazarov and Ormiston1993
Type species
Haplotaeniatum labyrintheum Nazarov and Ormiston, Reference Nazarov and Ormiston1993 (GIN 4679/33) from the Sakmarskaya Suite, Southern Urals by original designation.
Holotype
Specimen no. SEES/140703-55-HI1 (Fig. 11.8) and paratype SEES/140703-55-HI2 (digitized as Fig. 7 and Supplemental Data 4) in the micropaleontology collection of SEES-UQ. Known from Sandbian limestone beds in the Pingliang Formation, Guanzhuang section, Gansu Province, China.
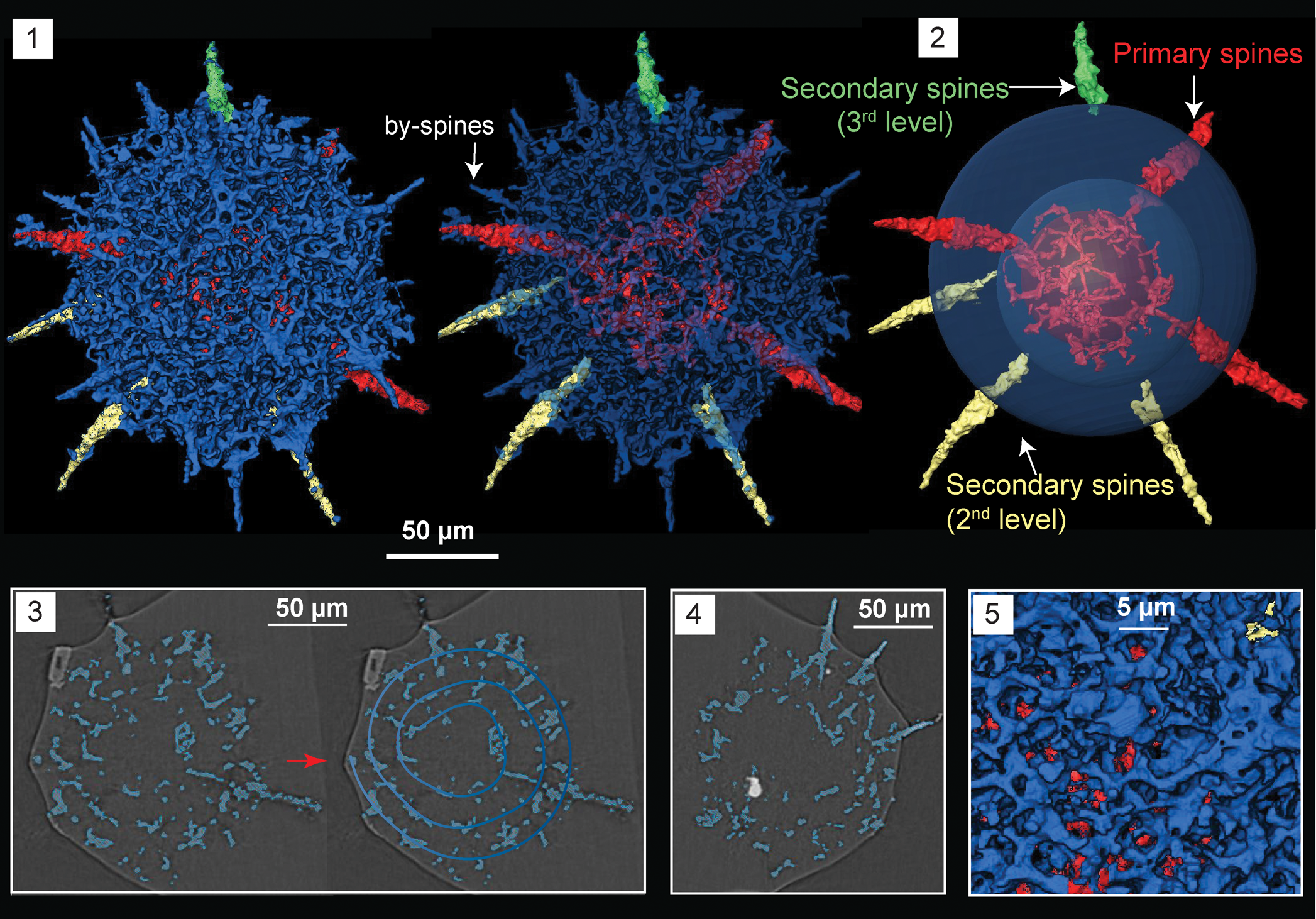
Figure 7. Structural details highlighted using micro-CT model of Haplotaeniatum implexa n. sp. (SEES/140703-55-HI2), paratype. Red and green spheres are inserted to aid visualization. (1) Complete skeleton; (2) configuration of spines and the layers from which they originate; (3) original micro-CT image and the interpreted spiraliform arrangement of layers; (4) growth of secondary spines from the mesh; (5) pseudospongy mesh of the outer shell surface. Scale bars are (1–4) 50 μm; (5) 5 μm.
Diagnosis
Haplotaeniatumidae characterized by numerous spines emanating from a dense, pseudospongy outer shell made of more than two spirally arranged discontinuous layers around an eccentric proloculus. Three tapering spines directly originate from the proloculus while the secondary spines initiate subsequently from the layers developing at different levels from the apophyses of the primary spines. Several thin by-spines occupy the outer surface.
Occurrence
Sandbian from China (herein).
Description
The spherical skeleton of H. implexa revealed by micro-CT observations constitutes a small proloculus overlain by a pseudospongy mesh composed of few incomplete layers arranged in a vague spiraliform configuration (Fig. 7.3). At least two levels existing in the entangled meshwork exposed when the points of origin of a few secondary spines were projected back using a digital model. Slightly eccentric proloculus (Ø = 62 μm) is made of thin bars that coalesce to form an approximately spherical shape. Consequently, large polygonal spaces appear on the proloculus surface. Three of the numerous spines emanating from the outer shell originate directly from the proloculus (Fig. 7.2). They are approximately arranged in a single plane and have a Ø = 15 μm at the base where they penetrate the outer surface. They develop apophyses that subsequently give rise to discontinuous layers of meshwork (Fig. 7.4). The layers themselves produce secondary spines of slightly lesser base Ø = 8–13 μm. Pseudospongy outer shell develops small pores up to Ø = 5 μm (Fig. 7.5) and several by-spines with lengths up to 40 μm. The average outer shell Ø = 180 μm (Fig. 7.1). All spines taper distally imposing a thorny appearance to the entire specimen.
Etymology
From the Latin ‘implexa’ symbolizing the ‘entangled.’
Remarks
Micro-CT reveals that layers of H. implexa are not as widely spread as those of Haplotaeniatum albaensis Perera, Aitchison, and Nothdurft, Reference Perera, Aitchison and Nothdurft2020 (see Perera et al., Reference Perera, Aitchison and Nothdurft2020). All the spherical Haplotaeniatum species encountered from the Darriwilian Shundy Formation (Pouille et al., Reference Pouille, Danelian and Maletz2014) are larger in outer diameter compared to H. implexa. However, it closely resembles the disorderly interwoven mesh of Haplotaeniatum sp. B (Pouille et al., Reference Pouille, Danelian and Maletz2014) and both share numerous outer spines. Haplotaeniatum sp. B is distinguished from H. implexa by its larger Ø = 313–320 μm and wider spine bases (Ø = 20–25 μm). The thin spines and their bases differentiate Haplotaeniatum sp. C (Pouille et al., Reference Pouille, Danelian and Maletz2014) from H. implexa, although the spongy meshwork of both appears similar. The shell dimensions of Haplotaeniatum sp. A (Yi et al., Reference Yi, Yuan, Aitchison and Feng2018) apparently matches with our specimen, but is too poorly preserved to comment further. Because the spiraliform pattern seen in H. implexa is less prominent, the origination points of spines can be traced to locate the exact positioning of levels in the spiraliform arrangement (see Fig. 7.2). The initial diagnosis of Haplotaeniatum only incorporated species with spiraliform or concentric shells (Nazarov and Ormiston, Reference Nazarov and Ormiston1993). However, subsequent revisions to the genus by Won et al. (Reference Won, Blodgett and Nestor2002) included species with a labyrinthine arrangement ranging between distinctly layered to entirely non-layered. The specimen encountered can be confidently placed within Haplotaeniatum and the micro-CT model provides insight into the microstructure of the genus.
Family Inaniguttidae Nazarov and Ormiston, Reference Nazarov and Ormiston1984, sensu Noble, Reference Noble1994, sensu Danelian and Popov, Reference Danelian and Popov2003
Genus Geminusphaera new genus
Type species
Geminusphaera kongtongensis n. sp. (SEES/140703-55-GK1) from the Pingliang Formation, in the Guanzhuang section Gansu Province, China.
Diagnosis
Inaniguttid radiolarians possessing two porous spherical shells and seven or more radially arranged primary spines originating from the inner sphere. Usually accompanied by several by-spines.
Occurrence
Sandbian from China (herein).
Etymology
Derived from Latin, ‘geminus-sphaera’ meaning ‘double-sphere.’
Remarks
Hitherto, generic classifications of members of the Inaniguttidae are based on a wide range of morphological features, including the number of spines, number of spheres, spacing between spheres, and their dimensions (Caridroit et al., Reference Caridroit, Danelian, O'Dogherty, Cuvelier, Aitchison, Pouille, Noble, Dumitrica, Suzuki, Kuwahara, Maletz and Feng2017; Noble et al., Reference Noble, Aitchison, Danelian, Dumitrica, Maletz, Suzuki, Cuvelier, Caridroit and O'Dogherty2017). The significance assigned to each of these aspects is still debated because some authors question their reliability after considering factors such as ontogeny and ecological stress (Noble and Aitchison, Reference Noble and Aitchison1995; Danelian and Popov, Reference Danelian and Popov2003; Suzuki, Reference Suzuki2006; Maletz, Reference Maletz2011; Trubovitz and Stigall, Reference Trubovitz and Stigall2018; Kachovich and Aitchison, Reference Kachovich and Aitchison2020). Micro-CT observation reveals the genus Geminusphaera n. gen. shares characteristics intermediate between Inanihella and Inanibigutta. Inanihella incorporates inaniguttids with two closely spaced porous spheres bearing numerous spines. The diagnosis of Inanihella further mentions the existence of an inner structure in the form of a spheroid (Ø = 45–55 μm) or its modification. This represents the initial skeleton of Inanihella. In addition, it is flexible toward forms with pylomes and furrowed main spines (Nazarov and Ormiston, Reference Nazarov and Ormiston1984; Nazarov, Reference Nazarov1988; Noble et al., Reference Noble, Aitchison, Danelian, Dumitrica, Maletz, Suzuki, Cuvelier, Caridroit and O'Dogherty2017). All these diagnostic features appear to be more compatible with Silurian morphotypes than those from the Ordovician (Nazarov and Ormiston, Reference Nazarov and Ormiston1984, Reference Nazarov and Ormiston1993; Siveter et al., Reference Siveter, Aitchison, Siveter and Sutton2007). However, several authors have assigned a few Ordovician species under Inanihella despite poor preservation that hinders precise identification. Inanihella (?) akzhala (Danelian and Popov, Reference Danelian and Popov2003) and I. bakanasensis Danelian and Popov, Reference Danelian and Popov2003 (reported by Danelian and Popov, Reference Danelian and Popov2003, and Maletz et al., Reference Maletz, Albanesi and Voldman2009) do not show any trace of an inner structure within the inner sphere. Inanihella penrosei (Ruedemann and Wilson, Reference Ruedemann and Wilson1936) described by Wang (Reference Wang1993) from the Pingliang Formation clearly shows the presence of a spheroid within the inner shell although the two spheres are not closely spaced as is typically seen in Silurian forms. Pouille et al. (Reference Pouille, Danelian, Ghobadi and Popov2013) assigned five species under Inanihella, of which only two have a spherical internal structure located within the two shells. Although the aforementioned encounters comply with the expected shell dimensions for Inanihella, the presence of an internal structure should be favored because it represents the initial skeleton. Failure to observe an initial skeleton because of poor preservation also suggests the possibility of the non-existence of any internal structure. Maletz and Bruton (Reference Maletz and Bruton2008) suggested the possibility of resorbing materials from inner spheres to form outer spheres during ontogeny. Meanwhile, Inanibigutta (Nazarov, Reference Nazarov1988) only recognizes two-sphered inaniguttids, with only six primary spines. Unlike Inanigutta, the number of spines on Inanibigutta is fixed (Noble et al., Reference Noble, Aitchison, Danelian, Dumitrica, Maletz, Suzuki, Cuvelier, Caridroit and O'Dogherty2017). The new genus Geminusphaera includes inaniguttids that are constructed from two distinct porous spheres and have seven or more primary spines.
Previous reports.—Maletz and Bruton (Reference Maletz and Bruton2008) reported an assemblage of 51 specimens that included inaniguttids with two to three spherical walls connected by beams and variable numbers of outer spines, which they referred to as Inanigutta sp. B. They suggested a revision to the Inaniguttidae, allocating all spumellarians with multiple spheres and spines under Inanigutta. Maletz and Bruton (Reference Maletz and Bruton2008, fig. 9F, G) illustrated two well-preserved specimens with two distinct spheres and multiple spines that could be incorporated within Geminusphaera n. gen. In these specimens, inner and outer sphere Ø = 140 μm and 70 μm, respectively, and the number of spines varies from 8–10. The description of characteristics of the mesh and the origination of spines are analogous to those of Geminusphaera n. gen., suggesting assignment to the new genus is appropriate. In combination, this extends the possible stratigraphic range of Geminusphaera n. gen. to the Darriwilian.
Helioentactinia? bakanasensis reported by Nazarov (Reference Nazarov1975) from Arenigian strata of the Ushkyzyl Formation (Zhylkaidarov, Reference Zhylkaidarov1999) was described from thin sections and has two shells and numerous spines. This material also could be allocated to the new genus because the sphere dimensions (115–169 μm, 86–131 μm) and species description align best with Geminusphaera n. gen. This would further extend the stratigraphic range of Geminusphaera n. gen. to at least the Lower Ordovician.
Geminusphaera grandis new species
Figure 11.11
Holotype
Specimen no. SEES/140703-55-GG1 (Fig. 11.11) in the micropaleontology collection of SEES-UQ. Known from Sandbian limestone beds in the Pingliang Formation, Guanzhuang section, Gansu Province, China.
Diagnosis
Two spherical shells connected by at least eight primary spines originating from the inner sphere. Outer shell has no by-spines.
Occurrence
Sandbian from China (herein).
Description
Spherical outer shell and inner shell show average Ø = 318 and 125 μm, respectively. Two spheres are separated by a gap of ~70 μm. Rounded to oval pores of varying sizes (8–24 μm) cover the outer shell, while the inner shell pores are generally smaller. Primary spines start as beams with constant Ø ≈ 13 μm and traverse through the outer shell. All the primary spines are equal in length (180 μm) and taper gradually before terminating with pointed ends. Maximum spine Ø of 25 μm is maintained at the surface intersection with the outer shell. Interpore frames and the bases of spines are attached smoothly through direct continuations.
Etymology
From the Latin ‘grandis’ meaning large.
Remarks
Previously, many double shelled, multiple spine bearing inaniguttids were assigned to the genus Inanihella, despite controversies discussed under genus remarks. This species is easy to distinguish from G. kongtongensis n. gen. n. sp. owing to its larger shell dimensions. In addition, pores of G. grandis n. gen. n. sp. are rounded to oval, whereas pores of G. kongtongensis n. gen. n. sp. exhibit irregular shapes.
Holotype
Specimen no. SEES/140703-55-GK1 (Fig. 11.12); SEES/140703-55-GK2 (digitized as Fig. 8 and Supplemental Data 5); and paratype, SEES/140703-55-GK3 (Fig. 11.7), in the micropaleontology collection of SEES-UQ. Known from Sandbian limestone beds in the Pingliang Formation, Guanzhuang section Gansu Province, China.
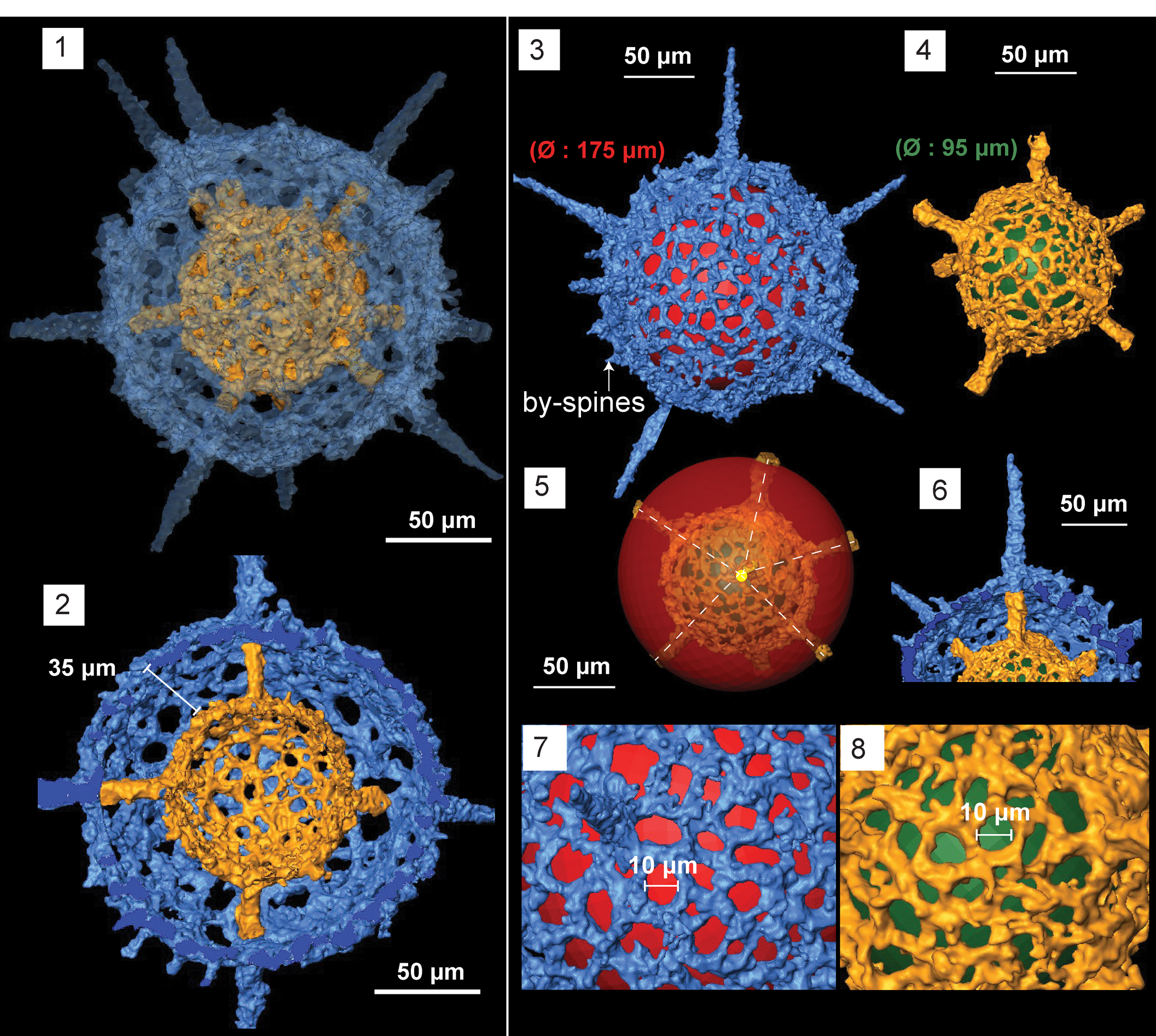
Figure 8. Structural details highlighted using micro-CT model of Geminusphaera kongtongensis n. gen. n. sp. (SEES/140703-55-GK2). Red and green spheres are inserted to aid visualization. (1) Complete skeleton with nine spines; (2) cross-section showing the empty inner shell; (3) outer shell; (4) inner shell; (5) radial arrangement of spines; (6) spines penetrating the outer sphere; (7) lattice framework of the outer shell; (8) lattice framework of the inner shell. Scale bars are (1–6) 50 μm; (7, 8) 10 μm.
Diagnosis
Inaniguttid radiolarians with two spherical latticed shells bearing 8–10 tapering primary spines originating from the inner shell that penetrate out through the outer shell. Several short conical by-spines are present.
Occurrence
Sandbian from China (herein).
Description
Micro-CT observation reveals two concentric spherical shells joined through nine radial beams originating at the surface of the inner sphere (Fig. 8.2). Outer sphere average Ø =175 μm whereas the inner sphere averages 90 μm (Fig. 8.3, 8.4). Where spines are not generated or penetrating, shell thickness is maintained at ~6 μm. Both spheres display similar lattice framework with sub-circular to irregular-shaped pores with Ø ranging from 6–15 μm (Fig. 8.7, 8.8). Except at junctions, bars delimiting the pores of the outer sphere maintain a regular thickness (4–5 μm), although this is inconsistent when it comes to the inner sphere. Two spheres are positioned ~35 μm apart (Fig. 8.2). The scanned specimen has nine primary spines, but the number ranges from 8–10. These primary spines originate at the surface of the inner sphere and rise as radial beams until they penetrate the outer sphere (Fig. 8.6). They gradually start to taper after piercing the outer surface and grow to a maximum length of 85 μm (measured from the surface of outer sphere). The width of the primary spines is ~15 μm at their base, which equals the Ø they maintain at the base of inner sphere. No apophyses are developed on the spines. Few short conical by-spines <10 μm are present on the outer shell surface. No trace of any internal structure beyond the inner shell.
Etymology
Named after the Kongtong District in China where the type locality is situated.
Remarks
The specimens encountered are well preserved and abundant enough to be able to determine shell measurements with confidence. Although primary spines are radially arranged and suggest the possible presence of a centrally located structure (Fig. 8.5), the micro-CT model clearly shows the vacant inner shell and how beams have developed from the inner shell surface. Oriundogutta miscella miscella (Nazarov in Nazarov and Popov, Reference Nazarov and Popov1980) reported from Pingliang Formation has similar dimensions (140–180 μm) to the outer sphere of G. kongtongensis n. gen. n. sp. and a similar number of primary spines (8–12). Illustrations in Wang (Reference Wang1993) are light-microscopic images, and it is possible that an inner sphere was undetected during observations. In addition, both reports come from the same formation, which may mean that both morphotypes represent a single species.
Genus Inanibigutta Nazarov and Ormiston in Nazarov, Reference Nazarov1988
Type species
Entactinosphaera aksakensis Nazarov, Reference Nazarov1975 (GIN 4333/1) from the Bestamak Formation, SW foothills of Chingiz Range, eastern Kazakhstan by original designation.
Inanibigutta pinglianensis Wang, Reference Wang1993
Figure 11.1
- Reference Wang1991
Inanibigutta pinglianensis Wang, p. 249, pl. 1, figs. 11, 12.
- Reference Wang1993
Inanibigutta pinglianensis; Wang, p. 100, pl. 5, figs. 1–8, pl. 6, figs. 1, 4.
- Reference Song, Cui, Hua and Wang2000
Inanibigutta pingliangensis [sic]; Song et al., p. 67, pl. 1, fig. 2.
Holotype
Specimen (NIGPAS-R0066) from Pingliang Formation, Gansu Province, China (Wang, Reference Wang1993, pl. 5, fig. 2).
Remarks
Specimens figured as Inanibigutta pingliangensis in Wang (Reference Wang1991), which were recovered from the Pingliang Formation, were illustrated and named but not accompanied by a species description. Wang (Reference Wang1993) again used probably the same specimen figured in his 1991 study, added some more specimens, and formally introduced s.l. pinglianensis with a name, proper species description, and a designated holotype.
Danelian and Popov (Reference Danelian and Popov2003) tentatively assigned Inanibigutta pinglianensis to Triplococcus, assuming the bifurcated apophyses on the outer shell are relics of a third shell. However, the specimen illustrated in Figure 11.1 clearly shows the bifurcated apophyses cannot be extended as a third shell because these sets of apophyses do not grow equidistant from the outer shell on all six spines. Micro-CT models of Triplococcus in Kachovich and Aitchison (Reference Kachovich and Aitchison2020) demonstrate its unique morphology, allowing differentiation between the disputed forms. The structure inside the inner shell remains uncertain because all of the encountered specimens had an infilled interior. Co-occurring fossils and zircons (An et al., Reference An, Zhang and Xu1985; Wu et al., Reference Wu, Li, Hu and Gong2014; Yang et al., Reference Yang, Chen, Kang, Ma, Chen, Tao and Deliang2020) indicate that the Zhaolaoyu Formation in Shaanxi Province, from which I. pinglianensis was reported for a second time (Song et al., Reference Song, Cui, Hua and Wang2000), is Late Ordovician, likely Sandbian age. Therefore, I. pinglianensis can be regarded as a good Sandbian marker taxon, especially in shallow water facies.
Inanibigutta verrucula (Nazarov and Popov, Reference Nazarov and Popov1976)
Figure 11.6
- Reference Nazarov and Popov1976
Entactinosphaera verrucula Nazarov and Popov, p. 408, fig. 1d.
- Reference Nazarov and Popov1980
Entactinosphaera verrucula; Nazarov and Popov, p. 38, pl. 3, fig. 6, pl. 11, fig. 6, text-fig. 17.
- Reference Nazarov1988
Inanibigutta verrucula; Nazarov, fig. 31.
- Reference Wang1993
Inanibigutta verrucula; Wang, p. 100, pl. 6, figs. 2, 3, 5–8.
- Reference Li1995
Inanibigutta verrucula; Li, p. 343, pl. 3, fig. 9.
- Reference Danelian and Clarkson1998
Inanibigutta verrucula; Danelian and Clarkson, p. 135, fig. 2g.
- Reference Danelian1999
Inanibigutta sp. cf. I. verrucula; Danelian, p. 630, fig. 4H.
- Reference Song, Cui, Hua and Wang2000
Inanibigutta aff. vurracula [sic]; Song et al., p. 67, pl. 1, fig. 6.
- Reference Danelian and Floyd2001
Inanibigutta (?) sp. O Danelian and Floyd, p. 493, fig. 4b.
- ?Reference Perera, Aitchison and Nothdurft2020
Inanibigutta sp. cf. I. verrucula; Perera et al., p. 2039, pl. 1, fig. h.
Holotype
Specimen (GIN 4333/18) from the Middle Ordovician, Bestamak Formation, SW foothills of Chingiz Range, eastern Kazakhstan (Nazarov and Popov, Reference Nazarov and Popov1976, fig. 1d).
Remarks
According to the original species description, one out of the six spines is generally lengthier than the rest. However, the closest specimens that displayed matching sphere dimensions did not show significant variation in spine length. In addition, none of the spines on the specimens maintains a constant Ø throughout their length (Nazarov and Popov, Reference Nazarov and Popov1976). Most spines on the observed specimens are broken at mid-length. Wang (Reference Wang1993) and Danelian and Clarkson (Reference Danelian and Clarkson1998) reported Ø = 180–260 μm and 131 μm for outer sphere Ø, for which Nazarov and Popov (Reference Nazarov and Popov1976) reported Ø = 160–200 μm. Because inner and outer sphere dimensions of the observed specimens (average Ø = 74 μm and 177 μm) correspond well with the measurements given in the original publication (75–85 μm; 160–200 μm), and all five specimens encountered show similar dimensions, they are assigned to I. verrucula.
Genus Inanigutta Nazarov and Ormiston, Reference Nazarov and Ormiston1984, sensu Nazarov, Reference Nazarov1988
Type species
Entactinia unica Nazarov, Reference Nazarov1975 (GIN 4333/2) from Bestamak Formation, SW foothills of Chingiz Range, eastern Kazakhstan by original designation.
Diagnosis
Internal structure represented by a hollow, non-porous sphere Ø = 40–75 μm, rarely less, with four to six rays emanating from it at angles of ~110–120°. A single, thick, mostly porous, envelope of up to Ø = 350 μm. Emended from Nazarov (Reference Nazarov1988).
Remarks
Inanigutta was established to include species with a single shell, demonstrating six-spine geometry (Nazarov and Ormiston, Reference Nazarov and Ormiston1984). However, the diagnosis was emended to include taxa with four to five spines by Nazarov and Popov (Reference Nazarov and Popov1980) who encountered two specimens with four-spine symmetry from an Upper Ordovician formation in Central Kazakhstan. Nevertheless, Inanigutta predominantly consists of six-spined species and no subsequent studies have reported forms with four/five spines.
Inanigutta complanata (Nazarov) Reference Nazarov1975
Figure 11.9
- Reference Nazarov1975
Entactinia complanata Nazarov, p. 56, pl. XV, figs. 11, 12, pl. XX, figs 7, 8.
- Reference Nazarov and Popov1980
Entactinia complanata; Nazarov and Popov, p. 29, pl. 1, figs. 2, 5, pl. 7, figs. 3, 4, pl. 11, figs. 3, 4, text-fig. 10.
- Reference Nazarov and Ormiston1984
Inanigutta complanata; Nazarov and Ormiston, pl. IV, fig. 1.
- ?Reference Goto, Umeda and Ishiga1992
Inanigutta cf. I. complanata Goto et al., p. 159, pl. 9, figs. 1–3.
- Reference Wang1993
Inanigutta complanata; Wang, p. 99, pl. 10, figs. 1, 2.
- Reference Danelian and Floyd2001
?Inanigutta complanata Danelian and Floyd, p. 493, fig. 4a.
- Reference Noble and Webby2009
Inanigutta complanata; Noble and Webby, pl. 5, figs. 10, 11, pl. 6, fig. 14.
- ?Reference Perera, Aitchison and Nothdurft2020
Inanigutta sp. cf. I. complanata; Perera et al., p. 2040, pl. 1, fig. i.
Holotype
Specimen (GIN 4333/29) from the Middle Ordovician, Bestamak Formation, SW foothills of Chingiz Range, eastern Kazakhstan (Nazarov, Reference Nazarov1975, pl. XX, fig. 8).
Remarks
Shell dimensions given in Nazarov and Popov (Reference Nazarov and Popov1980) are clearer than those in Nazarov (Reference Nazarov1975). With the exception of Wang (Reference Wang1993), no subsequent reports correspond exactly with original descriptions (outer shell; Ø = 190 μm and 175 μm, respectively, in Danelian and Floyd, Reference Danelian and Floyd2001, and Noble and Webby, Reference Noble and Webby2009). The dimensions given in Nazarov and Popov (Reference Nazarov and Popov1980) provide the basis for assignment of the observed specimens to I. complanata. Spine lengths of the studied specimens are slightly shorter, but the rest of the features align well within descriptions of Nazarov and Popov (Reference Nazarov and Popov1980). The structure of the initial skeleton from which the spines radiate is obscure.
Inanigutta gansuensis Wang, Reference Wang1993
Figure 11.14
- Reference Wang1993
Inanigutta gansuensis Wang, p. 99, pl. 7, figs. 1–8, pl. 8, figs. 1–11.
- Reference Pouille, Danelian, Ghobadi and Popov2013
Inanigutta gansuensis; Pouille et al., p. 1154, fig. 6.6.
- Reference Perera, Aitchison and Nothdurft2020
Inanigutta gansuensis; Perera et al., p. 2040, pl. 1, fig. d.
Holotype
Specimen (NIGPAS-R0087) from the Middle Ordovician, Pingliang Formation, Gansu Province, China (Wang, Reference Wang1993, pl. 7, fig. 1).
Remarks
Although specimens in this study exhibit similar sphere dimensions, they seem to have more secondary spines than the type specimens of I. gansuensis Wang. However, this variation could be an artefact of SEM illustrations. No specimens have spine lengths >300 μm, although Wang (Reference Wang1993) indicated a range of 200–500 μm.
Holotype
Specimen SEES/140703-49-IQ1 (Fig. 11.15) and SEES/140703-55-IQ2 (paratype, digitized as Fig. 9 and Supplemental Data 6) from Sandbian limestone beds in the Pingliang Formation, Guanzhuang section, Gansu Province, China.
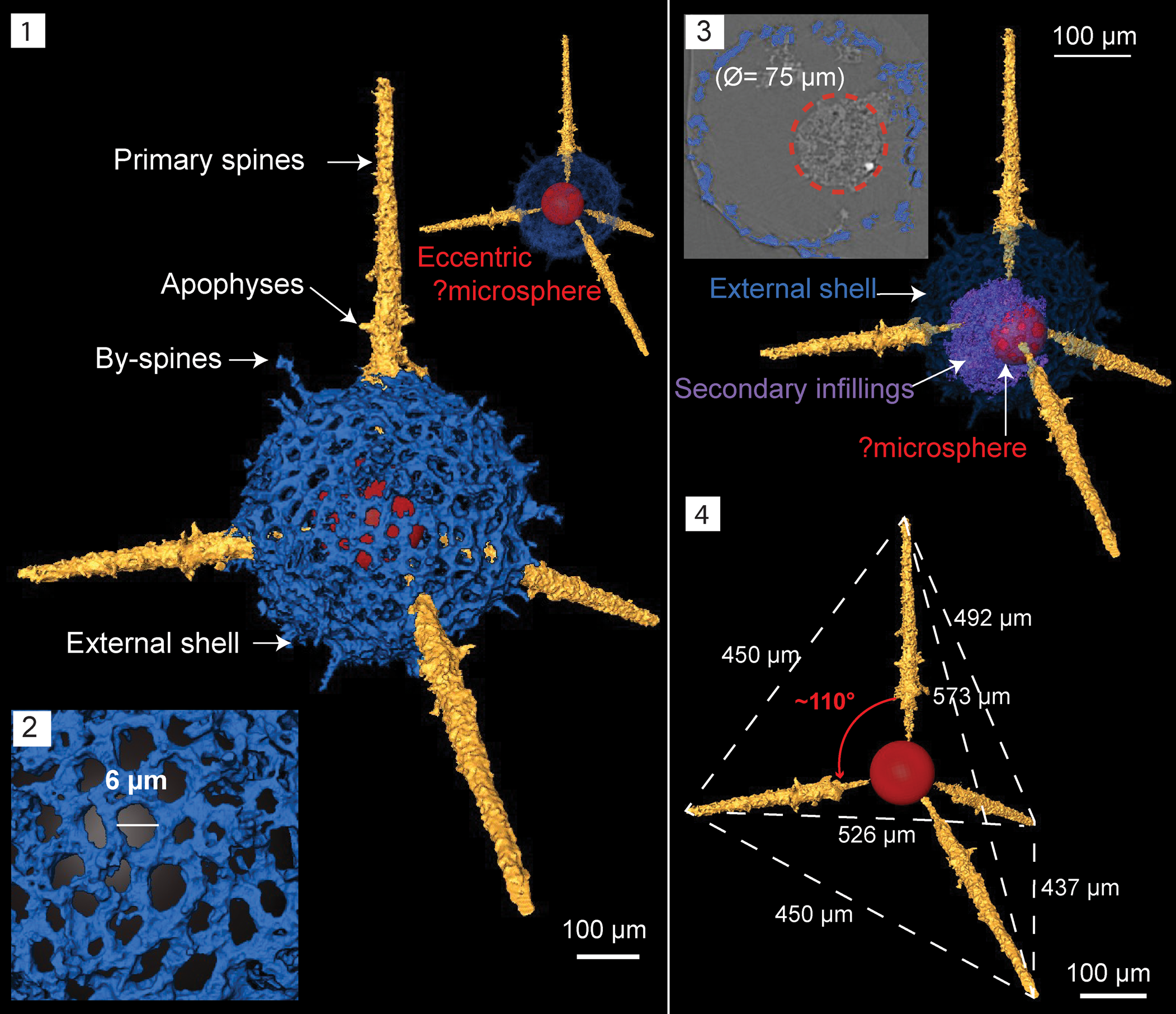
Figure 9. Structural details highlighted using micro-CT model of Inanigutta quadrispinosa n. sp. (paratype, SEES/140703-55-IQ2). Red sphere inserted to aid visualization. (1) Complete skeleton; (2) porous lattice of the outer shell; (3) ortho-slice showing the displaced ?microsphere and its present position within secondary infillings; (4) approximate tetrahedral arrangement and ?microsphere in its inferred original position. Scale bar is 100 μm in (1, 3, 4); 6 μm in (2).
Diagnosis
Inaniguttid radiolarians constructed with a single perfectly spherical lattice shell and four primary spines arranged at ~110° to one another. Primary spines are directed towards a centrally located initial structure, possibly a microsphere. Numerous conical by-spines are located on external surface.
Occurrence
Sandbian from China (herein).
Description
Micro-CT observations reveal the structural configuration of this inaniguttid is exclusively constructed with four spines and a single shell. A perfectly spherical external shell averages Ø = 190 μm and has a lattice arrangement with sub-circular to irregular-shaped pores (Fig. 9.1, 9.2). Maximum pore Ø ranges between 5–20 μm (Fig. 9.2). The surface of the external shell is occupied by numerous short conical by-spines up to 25 μm in length. Four rod-like primary spines with pointed tips penetrate the outer shell, maintaining an ~110° orientation to one another (Fig. 9.4). Primary spines originate from a slightly eccentrically located structure, perhaps a microsphere (Ø = 75 μm). Initial sections of spines lie within the shell with a Ø lesser than the outer sections and point towards the center attaining a long conical shape (Fig. 9.4). The Ø at the base of the primary spines on the shell surface is 20 μm and they extend a further 190–220 μm.
Etymology
From Latin ‘quadrispinosa’ referring to four spines.
Remarks
The structure of the internal element is unclear, although it vaguely suggests the presence of a microsphere. On orthoslices, the microsphere is dislocated and engulfed by secondary infillings (Fig. 9.3). The external shell is undamaged. Dimensions of the taxon described are similar to Inanigutta gansuensis Wang reported from the Pingliang Formation in the Guanzhuang section, although the latter has six rather than four spines. Wang (Reference Wang1993) described I. gansuensis using light-microscopic images and some of the documented specimens appear to have four-spine geometry. Inanigutta gansuensis also had been reported in two studies where SEM images were used for descriptions (Pouille et al., Reference Pouille, Danelian, Ghobadi and Popov2013; Perera et al., Reference Perera, Aitchison and Nothdurft2020). Both studies mention broken spines and do not report any six-spined specimens.
Genus Kalimnasphaera Webby and Blom, Reference Webby and Blom1986
Type species
Kalimnasphaera maculosa Webby and Blom, Reference Webby and Blom1986 (AM.F 134783) from limestone breccia deposits of the Malongulli Formation of New South Wales, Australia, by original designation.
Diagnosis
Palaeoactinommid with a combination of either reticulate inner shell, porous medullary shell, and pylomate cortical shell or one double-layered reticulate inner shell and pylomate reticulate cortical shell enveloped in a loosely fenestrate outer shell preserved in varying degrees. More than three long, gently tapering, primary and secondary spines may originate from any level of the shell. Few to numerous by-spines occupy the space on cortical shell and between cortical and fenestrate shells if present. A fenestrate outer shell is only complete in areas where lateral spinules are linked to by-spines and main spines. Emended from Webby and Blom, Reference Webby and Blom1986.
Remarks
Dunham and Murphy (Reference Dunham and Murphy1976) first reported Kalimnasphaera as ‘sphaeroids’ from Upper Ordovician calcareous concretions in Nevada. Material from that locality was revisited by Renz (Reference Renz1990) following a second encounter of the same genus from Upper Ordovician Australian limestones where it was formalized as Kalimnasphaera (Webby and Blom, Reference Webby and Blom1986). Kalimnasphaera has been reported several times from Australia (Goto et al., Reference Goto, Umeda and Ishiga1992; Umeda et al., Reference Umeda, Goto and Ishiga1992; Noble and Webby; Reference Noble and Webby2009), Kazakhastan (Pouille et al., Reference Pouille, Danelian, Ghobadi and Popov2013), and China (Cao et al., Reference Cao, Feng, Feng and Ling2014), although the last account is questionable. Cessipylarum Nazarov in Afanasieva, (Reference Afanasieva1986) and Cessipylorum Nazarov and Ormiston (Reference Nazarov and Ormiston1993) were later synonymized with Kalimnasphaera following revisions by Noble and Webby (Reference Noble and Webby2009), Pouille et al. (Reference Pouille, Danelian, Ghobadi and Popov2013), and Noble et al. (Reference Noble, Aitchison, Danelian, Dumitrica, Maletz, Suzuki, Cuvelier, Caridroit and O'Dogherty2017). However, micro-CT scans of Kalimnasphaera indicate substantial differences between the skeletal architectures of Kalimnasphaera and the diagnosis provided for Cessipylorum.
Kalimnasphaera pingliangensis new species
Figures 10, 11.4
- Reference Wang1993
Inanihella penrosei Wang, p. 102, pl. 1, figs. 2–5, pl. 2, figs. 1–9, pl. 3, figs. 1, 3, pl. 4, figs. 2, 3.
- Reference Webby and Blom1986
Kalimnasphaera? sp. Webby and Blom, pl. 3, fig. 10, pl. 4, fig. 1.
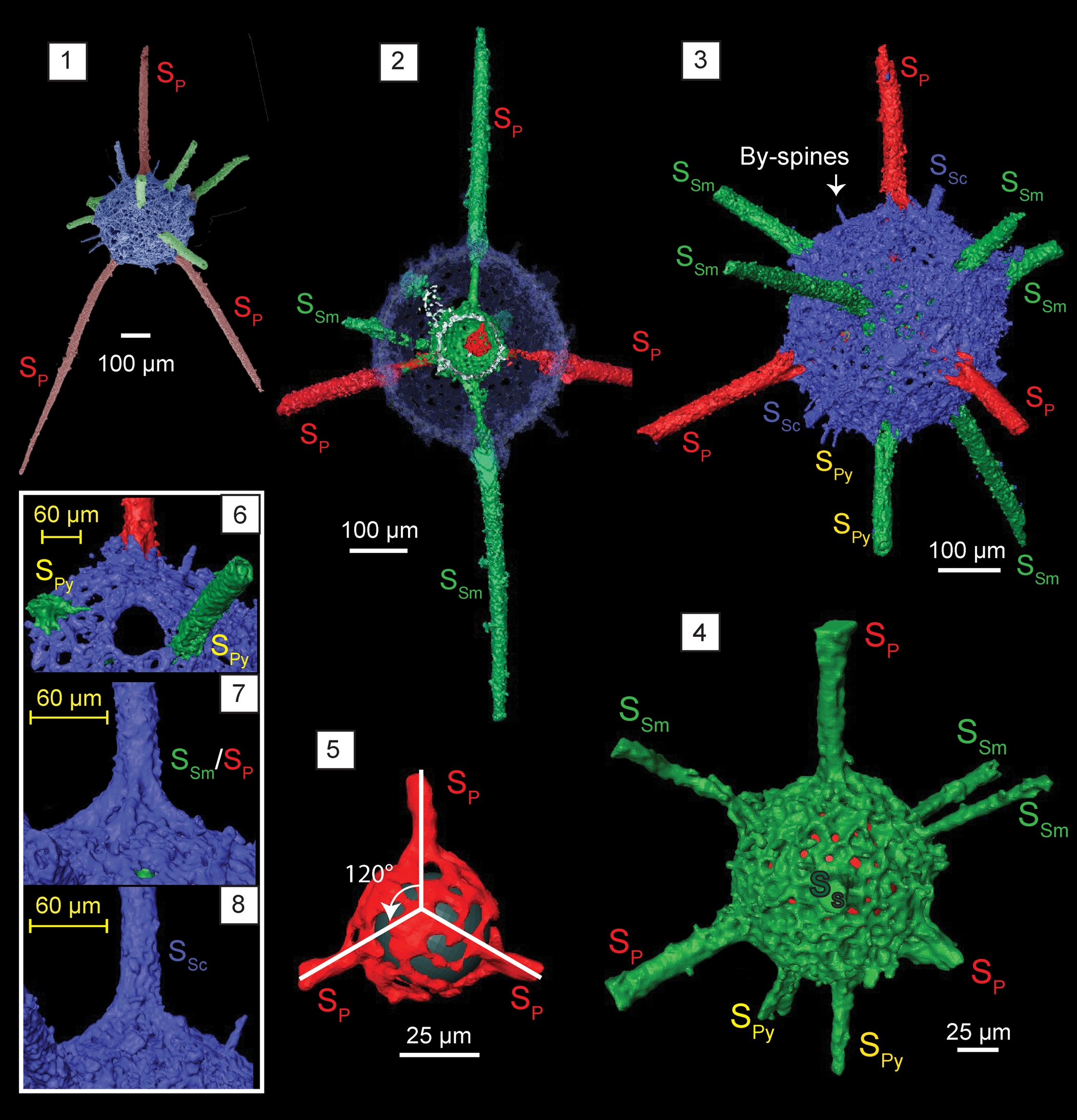
Figure 10. Structural details highlighted using micro-CT model of Kalimnasphaera pingliangensis n. sp. (paratype, SEES/140703-55-KP2). SP: primary spines initiating from microsphere. SSm: secondary spines initiating from medullary shell. SSc: secondary spines initiating from cortical shell. SPy: secondary spines associated with pylome. (1) SEM image, colorized, corresponding to micro-CT images; (2) cross section showing the arrangement of three spheres; (3) cortical shell and types of spines; (4) medullary shell; (5) microsphere and three primary spines (SP) originating from it (black sphere inserted to aid visualization); (6) pylome and the two associated spines (SPy); (7) primary or secondary spines supported with buttresses; (8) secondary spine (SSc) with a simple base initiating from the cortical shell. Scale bars are (1–3) 100 μm; (4, 5) 25 μm; (6–8) 60 μm.
Holotype
Specimen no. SEES/140703-55-KP1 (Fig. 11.4) and paratypes SEES/140703-55-KP2 (digitized as Fig. 10 and Supplemental Data 7) and SEES/140703-55-KP3 in the micropaleontology collection of SEES-UQ. Known from Sandbian limestone beds in the Pingliang Formation, Guanzhuang section, Gansu Province, China.
Diagnosis
Kalimnasphaerid lacking a fenestrate outer shell with few by-spines on the limited cortical shell surface. Pylomate cortical shell is perfectly spherical. Three primary spines originate directly from the inner reticulate microsphere and penetrate the single-layered medullary shell and cortical shell. The rest of the beams originate on the surface of the medullary shell and pass through the cortical shell forming secondary spines. At least six spines, including the three primary spines, develop buttresses on the cortical shell surface. Two secondary spines with simple bases guard a rimmed pylome on cortical shell.
Occurrence
Sandbian to Katian from China (Wang, Reference Wang1993, and herein) and Australia (Webby and Blom, Reference Webby and Blom1986).
Description
Observations using micro-CT reveal K. pingliangensis is exclusively constructed of three separate spheres interconnected by beams. The cortical shell (Ø = 325 μm) is porous, with sub-circular pores of Ø ranging from 6–20 μm (Fig. 10.3). Pore frame maintains a consistent thickness, except in areas with secondary recrystallizations. Medullary shell (Ø = 120 μm) is centrally located within the cortical shell (Fig. 10.4). It is generally imperforate despite having regularly arranged elevated polygonal-shaped pore frames. Random perforations can be seen between pore frames, but it is not clear whether this is an original feature. The innermost initial skeleton (Ø = 44 μm) is constructed with thin bars coalesced to form a microsphere along with three rod-like beams merging with the medullary shell (Fig. 10.5). The position of this microsphere within the medullary shell is uncertain (it appears to be dislocated in the scanned specimen). Three beams emanating from the microsphere develop at ~120° to one another (Fig. 10.5). They traverse through the medullary shell and emerge from the cortical shell forming rod-like primary spines with massive buttresses, which extend up to 840 μm in length (Fig. 10.7). Medullary shell gives rise to three secondary spines that penetrate the cortical shell with buttresses and few other secondary spines that have no significant base on the cortical shell. One or two secondary spines with simple bases originate from cortical shell itself (Fig. 10.8). Both primary and secondary spines have no apophyses. They taper distally, but remain as rods of Ø = 30 μm for most of their length. Some may bend slightly close to the base, while the rest are straight. Two secondary spines with regular bases are located in close proximity to the pylome, having a Ø ≈ 65 μm and fringed with an elevated rim (Fig. 10.6). Several conical by-spines on the cortical shell surface extend up to 50 μm.
Etymology
Named after the Pingliang Formation in China, where the type locality is situated.
Remarks
The species possesses a strong preservation bias compared to the rest of the assemblage. The absence of a fenestrate outer shell is characteristic because the primary, secondary, or by-spines had not developed apophyses to support lateral spinules as seen in K. maculosa. In addition, the medullary shell is not double-walled, as mentioned by Noble and Webby (Reference Noble and Webby2009). However, it should be noted that Webby and Blom (Reference Webby and Blom1986) recognized a “double walled medullary shell” as two separate shells with Ø = 13 μm and 40 μm. Over 20 specimens with good preservation were observed and none provided evidence for the existence of a fenestrate shell. Because K. pingliangensis n. sp. is a predecessor of K. maculosa, it is possible that the distinctly separate spheres seen in older forms may have combined at a later stage to form the double-walled, medullary-shelled morphotypes. However, Webby and Blom (Reference Webby and Blom1986, fig. 3.10) appears similar to K. pingliangensis, justifying its tentative assignment to K. maculosa. During the first study of the Pingliang Formation for radiolarians, Wang (Reference Wang1993) reported the presence of Inanihella penrosei. However, the figures in that paper clearly illustrate specimens of K. pingliangensis. Indeed, the wide range of dimensions Wang (Reference Wang1993) measured for elements of the shell, as well as an unmentioned third sphere, which can be clearly seen in Wang (Reference Wang1993, fig. 2.7) suggests assignment to I. penrosei was inappropriate.
Since Kalimnasphaera pingliangensis, which occurs in both N. gracilis and C. bicornis biozones, appears to be facies independent and may prove to be a useful index fossil alongside with Protoceratoikiscum spp., which is regarded as an index fossil for the Sandbian in the radiolarian biozonation of Aitchison et al. (Reference Aitchison, Suzuki, Caridroit, Danelian and Noble2017b). We note that K. pingliangensis occurs mostly in deep marine facies, such as cherts, which may explain its absence in an assemblage from a shallow-water, calcareous section.
Genus Oriundogutta Nazarov and Ormiston in Nazarov, Reference Nazarov1988
Type species
Astroentactinia ramificans Nazarov, Reference Nazarov1975 (GIN 4333/6) from Bestamak Formation, SW foothills of Chingiz Range, eastern Kazakhstan, by original designation.
Oriundogutta bella Wang, Reference Wang1993
Figure 11.2
- Reference Wang1991
Oriundogutta bella Wang, p. 249, pl. 1, fig. 14.
- Reference Wang1993
Oriundogutta bella Wang, p. 101, pl. 9, figs. 1–13.
- Reference Danelian1999
?Oriundogutta bella; Danelian, p. 632, pl. 5, fig. C.
Holotype
Specimen (NIGPAS-R0116) from Pingliang Formation, Gansu Province, China (Wang, Reference Wang1993, pl. 9, fig. 11).
Remarks
The specimen figured as Oriundogutta bella in Wang (Reference Wang1991), which was recovered from the Pingliang Formation, was not accompanied by any species description. Wang (Reference Wang1993) again used the same specimens from his 1991 study, adding some additional specimens, to formally introduce Oriundogutta bella with a proper species description and a designated holotype.
Only seven spines are visible. However, spine orientation suggests one other spine may be accommodated in the vacant surface towards lower-right position of the shell. The dimensions of shell elements correspond well with the holotype. The type material comes from older strata in the same section.
Oriundogutta ?miscella minuta Wang, Reference Wang1993
Figure 11.3
- Reference Wang1993
Oriundogutta miscella minuta Wang, p. 101, pl. 10, figs. 16, 17.
Holotype
Specimen (NIGPAS-R0135) from the Pingliang Formation, Gansu Province, China (Wang, Reference Wang1993, pl. 10, fig. 17).
Remarks
Poorly preserved single specimen shows slightly larger shell dimensions (shell Ø = 104 μm and spine length is ~54 μm) than that of the holotype (Ø = 80–100 μm and length of primary spines = 30–50 μm). The number of spines is not predictable, but appears to be more than eight. The specimen is thus tentatively assigned to O. ?miscella minuta.
Results
Radiolarians were extracted from samples SEES/140703-49 and SEES/140703-55, with the latter being stratigraphically higher. These samples yielded a diverse, well-preserved assemblage with 19 radiolarian species representing 12 genera. Faunal composition reflecting the taxonomic assignments and relative abundances in yielding samples are presented in Supplemental Data 8. Two new genera, Geminusphaera n. gen. and Protopylentonema n. gen., and six new species (Geminusphaera grandis n. gen. n. sp. Geminusphaera kongtongensis n. gen. n. sp., Haplotaeniatum implexa n. sp., Inanigutta quadrispinosa n. sp., Kalimnasphaera pingliangensis n. sp., and Protopylentonema ordosensis n. gen. n. sp.) are introduced. Although the Guanzhuang section is famous for graptolites, only SEES/140703-49 yielded recognizable taxa. Syndyograptus sinensis Mu, Reference Mu1963, and Amphigraptus divergens (Hall, Reference Hall1859), both of which have ranges in the C. bicornis Biozone (Chen et al., Reference Chen, Zhang, Wang, Goldman, Bergström, Fan, Finney, Chen, Chen, Bergström, Finney, Zhang, Fan, Chen, Goldman, Wang and Ma2017; Fig. 2), constrain the sample to the upper Sandbian. Conodonts also were recovered from the acid residues and include the short-ranging Sandbian forms Venoistodus venustus (Stauffer, Reference Stauffer1935) and Costiconus ethingtoni (Fåhraeus, Reference Fåhraeus1966) (Wang et al., Reference Wang, Bergström, Zhen, Chen and Zhang2013), constraining the radiolarian assemblage to a narrow window within the Baltoniodus gerdae subzone of the Amorphognathus tvaerensis Biozone. The long-ranging Sandbian taxon Periodon aculeatus was also recovered (see Fig. 2).
Discussion
Radiolarians are the only siliceous biomineralizing zooplankton known to have participated in the GOBE. The gradual or sudden increment of the taxonomic richness experienced by marine organisms, including radiolarians, during Early to Middle Ordovician is recognizable in many diversity studies conducted regardless of the region (Stigall et al., Reference Stigall, Edwards, Freeman and Rasmussen2019; Danelian and Monnet, Reference Danelian and Monnet2021). However, encounters of radiolarians during the GOBE are limited, and might indicate an ecological preference of early Paleozoic radiolarians over preservation biases. Various paleogeographic reconstructions for Late Ordovician time offer various scenarios, each backed by a variety of geophysical and paleontological evidence that show positions of Kazakh terranes, South and North China, and the Australian sector of Gondwana, all near or adjacent to the equator. The relative distribution of positions for the central and eastern Asian blocks continues to be a topic of on-going debate (Cocks and Torsvik, Reference Cocks and Torsvik2020). These adjacent terranes account for a majority of Late Ordovician radiolarian occurrences: Malongulli and Ballast formations, Australia (Webby and Blom, Reference Webby and Blom1986; Goto and Ishiga, Reference Goto and Ishiga1991; Goto et al., Reference Goto, Umeda and Ishiga1992; Iwata et al., Reference Iwata, Schmidt, Leitch, Allan and Watanabe1995; Noble and Webby, Reference Noble and Webby2009); Pingliang and Zhaolaoyu formations, North China (Wang, Reference Wang1993; Song et al., Reference Song, Cui, Hua and Wang2000; Wang et al., Reference Wang, Cheng and Zhang2010); Wufeng Formation, South China (Wang and Zhang, Reference Wang and Zhang2011; Zhang et al., Reference Zhang, Luo and Zhang2018); and Ulkuntas Horizon, Kazakhstan (Nazarov and Popov, Reference Nazarov and Popov1980), reported to date. The rest lie in the Laurentian and Baltic paleo-shelves in the Hanson Creek Formation, Nevada (Dunham and Murphy, Reference Dunham and Murphy1976; Renz, Reference Renz1990), and Saunja Formation, Estonia (Nazarov and Nolvak, Reference Nazarov and Nolvak1983), respectively. Radiolarian assemblages reported from all these isolated paleo-geographical sites show unique faunal compositions, despite sharing some genera and species. The distribution of known Late Ordovician radiolarian occurrences on a paleo-geographical map highlights their concentration in areas of low latitude (Fig. 13). Many researchers estimate temperatures were close to modern-day, equatorial sea surface temperatures (25–32°C) around low paleo-latitudes (up to 40°N/S) during Late Ordovician time (Trotter et al., Reference Trotter, Williams, Barnes, Lécuyer and Nicoll2008; Song et al., Reference Song, Wignall, Song, Dai and Chu2019; Cocks and Torsvik, Reference Cocks and Torsvik2020). The propensity of Late Ordovician radiolarians to flourish in this range of sea surface temperatures is a characteristic trend shared by other marine clades. Kröger (Reference Kröger2018) estimated that peak diversities for all marine biota of the Late Ordovician were concentrated around 0–30° paleo-latitudes, whereas Early to Middle Ordovician peaks lay within zones between 15–45°.
Figure 13. Global distribution of known late Ordovician radiolarian localities displayed on a paleogeographic reconstruction model redrawn from Cocks and Torsvik (Reference Cocks and Torsvik2020, fig. 6). B: Baltica, G: Gondwana, L: Laurentia, S: Siberia.
Despite sharing similar SSTs, abiotic factors, such as different levels of dissolved oceanic Si and shelf environment changes, triggered by fluctuating sea levels might have had a cumulative effect at local and global scales, possibly causing the diversity differences among radiolarian assemblages in different micro-continents. While the levels of dissolved silica in oceans control the biomineralization of radiolarian skeletons, speciation within a population is naturally promoted by geographic barriers and entering of members from an adjacent niche, which could occur as results of intermittent sea-level changes (Kidder and Tomescu, Reference Kidder and Tomescu2016; Stigall et al., Reference Stigall, Bauer, Lam and Wright2017).
The Katian Hanson Creek Formation in Nevada (Dunham and Murphy, Reference Dunham and Murphy1976; Renz, Reference Renz1990) and Malongulli Formation in Australia (Webby and Blom, Reference Webby and Blom1986; Goto et al., Reference Goto, Umeda and Ishiga1992; Noble and Webby, Reference Noble and Webby2009) host the most diverse radiolarian assemblages, and genera representing all four orders (Albaillellaria, Archaeospicularia, Entactinaria, and Spumellaria) have been reported to have existed at the time. Those two assemblages share many species, although the Laurentia and Australian sectors of Gondwana were geographically remote from one another during the Katian. In contrast, the Wufeng Formation in the South China block, positioned relatively near the slightly older high-diversity terrane of Australia, contained a less-diverse assemblage dominated by inaniguttids. These upper Katian–Hirnantian assemblages on the shelves of the South China block might have been influenced by the dominating population of inaniguttids dispersed in the adjacent North China block during the Sandbian. The neighboring shelves of the Kazakh terranes, which were a hotspot of radiolarians during the Middle Ordovician, have only a single report of Late Ordovician radiolarians. Only the Baltic radiolarian assemblage reported to date is coeval with our study, and it is the assemblage located farthest from the paleo-equator. Neither the abundance nor preservation of both Kazakh and Baltic assemblages reported several decades ago is sufficient to remark on their diversity, despite sharing H. baltica, a rare species that was also found in the Pingliang Formation. Given the preference of Late Ordovician radiolarians for warm waters, the drop of SSTs that accompanied the Hirnantian glaciation (Trotter et al., Reference Trotter, Williams, Barnes, Lécuyer and Nicoll2008; Cocks and Torsvik, Reference Cocks and Torsvik2020) might have negatively affected radiolarian diversity and abundance.
During the last phase of GOBE in the Late Ordovician, most of the marine clades, including radiolarians, started to adapt to the new equilibrium they reached with their ecosystems. The response of radiolarians mainly included structural adaptations, such as development of a pylome that was initiated by Darriwilian time in Triplococcus, Westernbrookia, and Protopylentonema n. gen., and successfully passed on to Late Ordovician Kalimnasphaera, Borisella, and Sandbian members of Protopylentonema n. gen. By Late Ordovician time, bandage-type shell wall-bearing antygoporids and aspiculids of the Middle Ordovician were mostly replaced by solid, thick skeleton-bearing inaniguttids (Kalimnasphaera, Geminusphaera n. gen., Inanibigutta, Inanigutta, and Oriundogutta), increasing the robustness of the skeleton and adapting to both shallow- and deep-water facies. Although radiolarian discoveries are usually subjected to strong preservation and collection biases, which shadows true relative species abundances, many studies conducted for Ordovician radiolarians confirm the dominating occurrence of inaniguttids (Nazarov, Reference Nazarov1988; Wang, Reference Wang1993; Danelian and Clarkson, Reference Danelian and Clarkson1998; Danelian and Floyd, Reference Danelian and Floyd2001; Maletz and Bruton, Reference Maletz and Bruton2008; Maletz et al., Reference Maletz, Albanesi and Voldman2009; Noble and Webby, Reference Noble and Webby2009; Kachovich and Aitchison, Reference Kachovich and Aitchison2020; Perera et al., Reference Perera, Aitchison and Nothdurft2020). This indicates that the Inaniguttidae were the most successful family to diversify during the GOBE.
Since the GOBE is recognized as a speciation event, the identification of phylogenetic differences in the fossil skeleton is crucial. The establishment of all six new species and two new genera in this study was supported with use of micro-CT imagery after considering their initial skeleton structure rather than skeletal dimensions. Since the Family Inaniguttidae was established, its definition has undergone multiple revisions and emendations because of the abundance of taxa and high degree of morphological variations seen in their skeletons and/or dimensions even among coeval encounters. Therefore, a diagnosis that emphasizes the nature of the initial skeleton and accompanying primary-level skeletal features, such as spines originating from the initial skeleton (primary spines) and number of shells directly developing from apophyses of primary spines, is required. If those fundamental elements are preferred over the dimensions used for species diagnosis, it is less likely to be affected by ontogenetic change and adaptation to ecological stress conditions, which may cause major phylogenetic misinterpretations.
Acknowledgments
The research was financially supported by the Australian Research Council grant no. ARC DP 1501013325 (to JCA). We thank C. Evans at the Julius Kruttschnitt Mineral Research Centre, UQ, for conducting micro-CT measurements of the radiolarian specimens. S. Kachovich (UQ) and Z. Yan (CAGS) collected the samples. We also thank journal Editor B. Hunda, Associate Editor P. Noble, and reviewers L. Hui and T. Danelian for their constructive comments that have helped to improve the manuscript. This paper is a contribution to the International Geoscience Programme (IGCP) Project 653, The Onset of the Great Ordovician Biodiversification Event.
Data availability statement
Data available from the Dryad Digital Repository: http://doi.org/10.5061/dryad.gtht76hn2.