Introduction
The hemipteran superfamily Cercopoidea Leach, Reference Leach1815, known as froghoppers or spittlebugs, comprises nearly 3000 described species. The high-level classification of living Cercopoidea is still controversial: five modern cercopoid families (Cercopidae Leach, Reference Leach1815, Aphrophoridae Amyot and Serville, Reference Amyot and Serville1843, Clastopteridae Dohrn, Reference Dohrn1859, Machaerotidae Stål, Reference Stål1866, and Epipygidae Hamilton, Reference Hamilton2001) have been described to date, but various taxonomists recognize three to five (Hamilton, Reference Hamilton2001, Reference Hamilton2012; Dietrich, Reference Dietrich2002, Reference Dietrich2005; Holzinger et al., Reference Holzinger, Kammerlander and Nickel2003). Moreover, three extinct families from the Mesozoic have been attributed to this superfamily (Wang et al., Reference Wang, Szwedo and Zhang2012; Chen et al., Reference Chen, Wang, Zhang, Wang and Zheng2015a).
The family Procercopidae Handlirsch, 1906, recorded from the Early Jurassic to Early Cretaceous in Germany, Russia, Central Asia, Southeast Asia, and China, is widely accepted as the stem group of cercopoids (Shcherbakov and Popov, Reference Shcherbakov and Popov2002; Chen et al., Reference Chen, Wang, Zhang, Wang and Zheng2015a). Representatives of the extinct froghopper families Sinoalidae Wang and Szwedo in Wang et al., Reference Wang, Szwedo and Zhang2012 and Cercopionidae Hamilton, Reference Hamilton1990 were exclusively known from the Jurassic deposits in northeastern China and the Early Cretaceous of Brazil, respectively (Hamilton, Reference Hamilton1990; Wang et al., Reference Wang, Szwedo and Zhang2012). By the mid-Cretaceous, primitive cercopoids became extinct and ancestors of modern groups appeared and became diversified (Shcherbakov and Popov, Reference Shcherbakov and Popov2002; Chen et al., Reference Chen, Wang, Zhang, Wang and Zheng2015a).
The family Sinoalidae was erected as part of the latest Middle–earliest Late Jurassic Daohugou Biota, Inner Mongolia of China. Sinoalidae, which is closely related to early Procercopidae and shares some plesiomorphic characters with ancient Hylicelloidea, represents one of the distinct diversifications of ancestral Cercopoidea. Up to now, five genera (Luanpingia Hong, Reference Hong1983, ?Hebeicercopis Hong, Reference Hong1983, Huabeicercopis Hong, Reference Hong1983, Sinoala Wang and Szwedo in Wang et al., Reference Wang, Szwedo and Zhang2012, and Jiania Wang and Szwedo in Wang et al., Reference Wang, Szwedo and Zhang2012) from the Jurassic deposits of northeastern China have been attributed to this distinct froghopper family (Fig. 1; Wang et al., Reference Wang, Szwedo and Zhang2012).
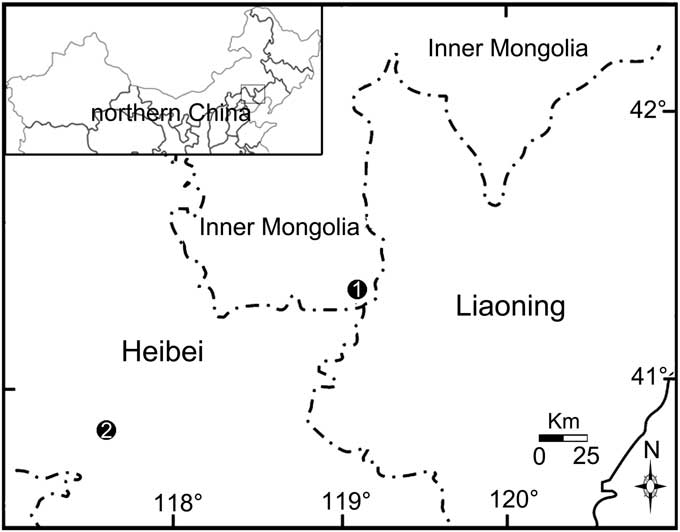
Figure 1 Locations of fossil specimens of the family Sinoalidae. 1, Daohugou, Ningcheng County, Inner Mongolia; 2, Zhouyingzi, Luangping County, Heibei.
We herein report some fossil sinoalids from the Daohugou Biota. A new genus and species with some significant morphological traits different from known con-familial taxa is established, and the family is revised further based on the new fossil specimens.
Materials and methods
The new Mesozoic sinoalids described herein were collected from the well-known Daohugou fossil-bearing strata of northeastern China (Fig. 1; Wang, Reference Wang2009). Daohugou is now considered to be one of the most important insect Lagerstätten (Rasnitsyn et al., Reference Rasnitsyn, Zhang and Wang2006) and has yielded abundant and diverse insects (e.g., Wang and Zhang, Reference Wang and Zhang2011; B. Wang et al., Reference Wang, Zhang, Jarzembowski, Fang and Zheng2013; Chen et al., Reference Chen, Wang, Engel, Wappler, Jarzembowski, Zhang, Wang, Zheng and Rust2014; Yan et al., Reference Yan, Wang, Ponomarenko and Zhang2014). Generally, the fossil-bearing beds at Daohugou were placed within the Jiulongshan Formation of Bathonian–Callovian (late Middle Jurassic). However, recent isotopic dating results indicated that the Daohugou beds were deposited in the geological age of 164–158 Ma (Liu et al., Reference Liu, Liu and Zhang2006, Reference Liu, Kuang, Jiang, Peng, Xu and Sun2012; L. Wang et al., Reference Wang, Hu, Zhang, Zhang, He, Deng, Wang, Zhou and Zhu2013), which is Callovian–Oxfordian (latest Middle–earliest Late Jurassic) according to the updated International Chronostratigraphic Chart (Cohen et al., Reference Cohen, Harper and Gibbard2016).
Nel et al. (Reference Nel, Prokop, Nel, Grandcolas, Huang, Roques, Guilbert, Dostal and Szwedo2012) proposed a new interpretation of wing venation pattern for all Paraneoptera, assuming that CuA gets fused with M+R stem at the wing base and connected with CuP by a specialized crossvein cua-cup after its departure from M+R, which is remarkably different from the traditional interpretations. The venational terminologies used herein follow Nel et al. (Reference Nel, Prokop, Nel, Grandcolas, Huang, Roques, Guilbert, Dostal and Szwedo2012).
The fossil sinoalids were examined dry or under alcohol, with details observed and photographed under a stereomicroscope (ZeissSteREO Discovery V8). Line drawings were prepared with CorelDraw 12.0 and Adobe Photoshop CS3.
Repository and institutional abbreviation
All the material and figured specimens in this study are deposited in Shandong Tianyu Museum of Nature (STMN), Pingyi, Shandong Province, China.
Systematic paleontology
Order Hemiptera Linnaeus, Reference Linnaeus1758
Suborder Cicadomorpha Evans, Reference Evans1946
Superfamily Cercopoidea Leach, Reference Leach1815
Family Sinoalidae Wang and Szwedo in Wang et al., Reference Wang, Szwedo and Zhang2012
Diagnosis (emended)
Forewing with apices of costal area and clavus almost at the same level; costal area and/or clavus more sclerotized and punctate and remaining parts membranous; Pc+CP long and thicken, almost parallel to costal margin; M two-branched. Hind wing with M unbranched; crossvein m-cua basad of crossvein r-m. Three ocelli rather than two. Hind tibia with two rows of lateral spines (four at most in number for each row).
Remarks
Our new fossil materials suggest that the relative branching position of M and CuA is variable in Sinoalidae and so not appropriate for family-level diagnosis. In addition, the fossils reported herein also provide some information on morphological diversity and evolution of hind wings and hind tibiae of the family (see Discussion).

Genus Shufania new genus
Type species
Shufania hani n. gen. n. sp., by present designation and monotypy.
Diagnosis
As for the type species.
Etymology
The generic name, Shufania, a feminine noun derived after Prof. Shufan Han, a well-known artist and the curator of the Museum of Linyi University.
Shufania hani new species
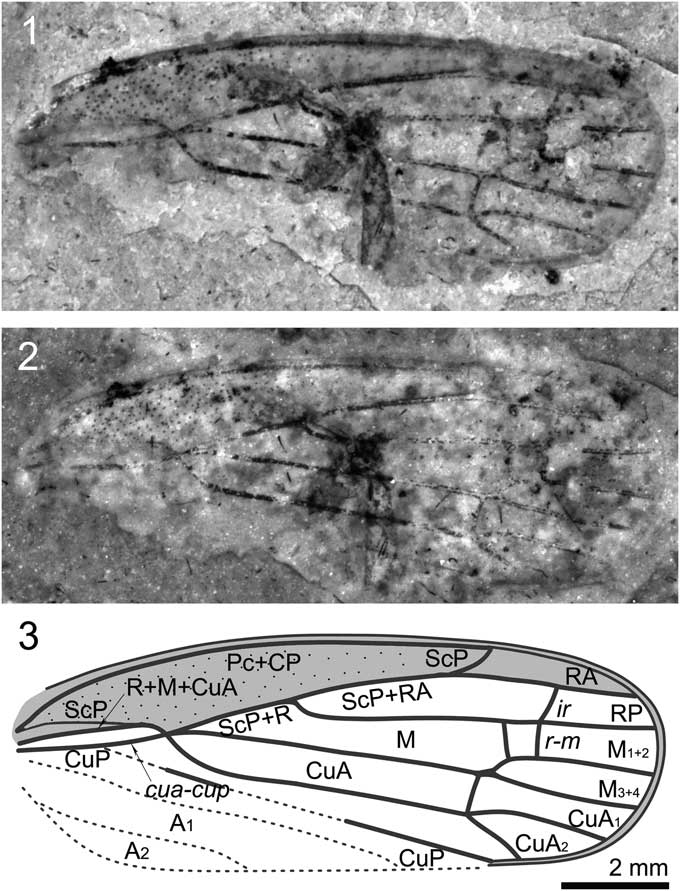
Figure 2 Shufania hani n. gen. n. sp.: (1) holotype STMN48-1807; (2) holotype under alcohol; (3) illustration of holotype. Scale bar for all images.
Holotype
STMN48-1807, sex unknown, a sole forewing with clavus missing.
Diagnosis
Forewing small, basal cell long, about one-fourth as long as forewing length; ScP+R+M+CuA bifurcating into ScP+R+M and CuA at junction with crossvein cua-cup stem ScP+R+M extremely short; ScP+RA ~1.5 times as long as stem ScP+R; base of RP strongly curved; M very long and nearly straight, branched into M1+2 and M3+4 at about basal 0.75 wing length; crossvein m-cua connecting to stem M basad of bifurcation of M; CuA branching into CuA1 and CuA2 at junction with crossvein m-cua, slightly basad of bifurcation of M.
Occurrence
This specimen was collected from Daohugou Village (41°18′N, 119°13′E), Shantou Township, Ningcheng County of Inner Mongolia, China. The fossil bed is Callovian–Oxfordian (latest Middle–earliest Late Jurassic) in age.
Description
Specimen STMN48-1807 (Fig. 2): Forewings length ~11.6 mm long, width as preserved ~4.2 mm. Costal margin slightly arched. Apical margin rounded. Costal area punctate, long and narrow, with length/width ratio ~6.0. Apices of costal area and clavus almost at the same level, at ~0.75 of wing length. Basal cell long, about one-quarter as long as forewing length. Apical cells six. Pc+CP long and thicken, almost parallel to costal margin. ScP weak and short, running to and fusing with R+M+CuA at ~0.2 of wing length, separating from the latter and then ending at apex of costal area. ScP+R+M+CuA bifurcating into ScP+R+M and CuA at junction with crossvein cua-cup, near basal quarter of wing length; stem ScP+R+M extremely short. ScP+RA ~1.5 times as long as stem ScP+R; R bifurcating into RA and RP in basal 0.45 wing length; RA and RP unbranched, connected to each other by crossvein ir; RA slightly arched, RP strongly curved at base and then nearly straight. Crossvein r-m two; basal one slightly oblique; apical one nearly vertical and connecting to RP at junction of RP and crossvein ir. Stem M very long and nearly straight, branched into M1+2 and M3+4 at about basal 0.75 wing length, just distad of the apices of costal area and clavus; M1+2 and M3+4 unbranched. Crossvein im invisible; crossvein m-cua connecting to stem M instead of branch M1+2. Stem CuA strongly curved at base, and then nearly straight, branching into CuA1 and CuA2 at junction with crossvein m-cua, slightly basad of bifurcation of M. CuP largely destroyed, as preserved straight. A1 and A2 completely missing.
Etymology
The species is named after Prof. Shufan Han.
Remarks
The genus undoubtedly belongs to the family Sinoalidae on the following characteristics: costal area punctate; apical cells six; Pc+CP long and thicken, almost parallel to costal margin; ScP weak and short; RA and RP unbranched; M with two terminal branches. Shufania n. gen., however, distinctly differs from all known sinoalids in having forewing with crossvein m-cua connecting to stem M, bifurcation of M distad of apices of costal area and clavus, CuA branching into CuA1 and CuA2 at junction with crossvein m-cua, slightly basad of bifurcation of M. In addition, the new taxon differs from Jiania Wang and Szwedo, 2012 in possessing a smaller forewing; from Sinoala Wang and Szwedo, 2012, Luanpingia Hong, Reference Hong1983, and Huabeicercopis Hong, Reference Hong1983 in possessing a forewing with a very short stalk M+R.
Genus Jiania Wang and Szwedo in Wang Szwedo, and Zhang, Reference Wang, Szwedo and Zhang2012
Jiania gracila Wang and Szwedo in Wang Szwedo, and Zhang, Reference Wang, Szwedo and Zhang2012
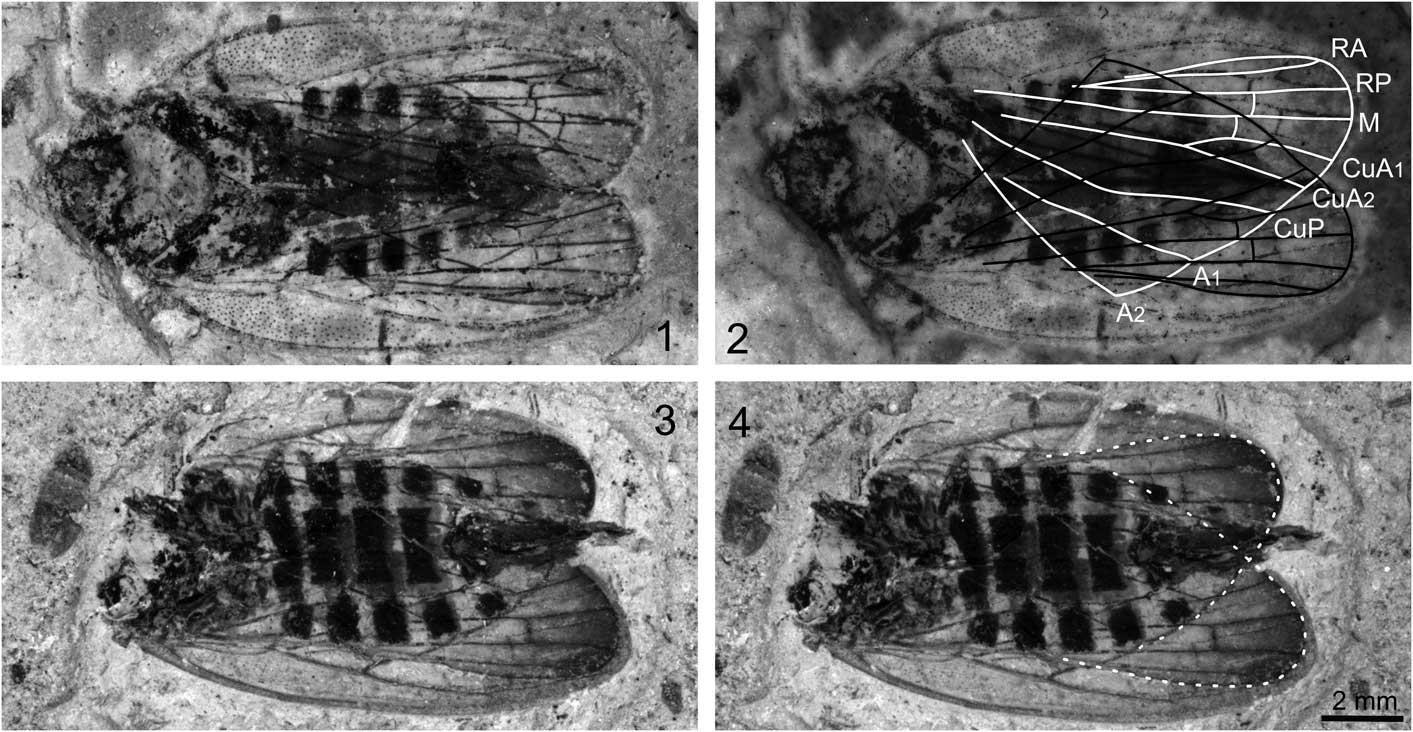
Figure 3 Jiania gracila Wang and Szwedo in Wang et al., Reference Wang, Szwedo and Zhang2012: (1) specimen STMN48-1809; (2) specimen STMN48-1809 under alcohol, with line drawings of hind wings; (3) specimen STMN48-1808; (4) specimen STMN48-1808 with outline of hind wings. Scale bar for all images.
2012 Jiania gracila Wang and Szwedo in Wang et al., p. 1237, figs. 3E, 4D, 4E, 7C, 9.
2013 Jiania gracila; Li et al., p. 7, fig. 5.
Diagnosis
Forewing ~3.1–3.6 times as long as wide; C3 ~1.5 times as long as adjoining apical cell; ScP+R+M+CuA bifurcating into ScP+R+M and CuA at junction with crossvein cua-cup, near basal one-fifth wing length; stalk ScP+R+M present, extremely short. Hind wing with distal part darkly stained; R bifurcating into RA and RP at base of ending point of A2; CuA and CuA1 at 145° angle; CuA and CuA2 in alignment.
Materials
STMN48-1808, female, adult in dorsoventral aspect with wings preserved at the top of the body, head missing; STMN48-1809, female, adult in dorsoventral aspect with wings preserved at the top of the body, nearly complete.
Occurrence
The two specimens were collected from Daohugou Village (41°18′N, 119°13′E), at the same locality where the holotype was collected.
Description
Specimen STMN48-1809 (Fig. 3.1, 3.2): body 15.2 mm long including forewings in repose. Head with compound eyes length as preserved 1.5 mm, obviously narrower than pronotum. Pronotum ~1.6 mm long and 3.5 mm wide, anterior margin straight, posterior margin poorly preserved. Mesonotum ~3.1 mm long in midline and ~4.0 mm wide at base. Abdomen with ovipositor ~8.3 mm long and ~4.7 mm wide, with seven segments visible. Ovipositor slightly extending beyond forewing tips.
Forewings slender, ~13.0 mm long, 4.2 mm wide, with length/width ratio ~3.1. Costal margin slightly arched. Posterior margin almost straight. Apical margin rounded. Costal area punctate, long and narrow, with length/width ratio ~6.0. Clavus strongly arched, long, with length/width ratio ~5.2. Apices of costal area and clavus almost at the same level, at ~0.7 of wing length. Apical cells six. Pc+CP long and thicken, almost parallel to costal margin. ScP weak and short, running to and fusing with ScP+R+M+CuA, separating from the latter and then ending at apex of costal area. ScP+R+M+CuA bifurcating into ScP+R+M and CuA at junction with crossvein cua-cup, near basal one-fifth wing length; stem ScP+R+M extremely short. R bifurcating into RA and RP in basal 0.4 wing length; RA and RP unbranched, connected to each other by crossvein ir; RA slightly arched, RP sinuous and curved at junction with crossvein ir and r-m. Stem M nearly straight, branched into M1+2 and M3+4 slightly distad of middle of wing (at about basal 0.55 wing length); M1+2 and M3+4 unbranched, connected to each other by crossvein im. Cell C3 ~1.5 times as long as adjoining apical cell. Stem CuA strongly curved at base, and then nearly straight, branching into CuA1 and CuA2 at basal 0.68 wing length. CuP strongly curved at junction with crossvein cua-cup, and then nearly straight. A1 and A2 poorly preserved.
Hind wing slightly shorter than forewing, with distal portion darkly stained, without peripheric membrane. Stem R bifurcating RA and RP at base of ending point of A2. Vein M unbranched, nearly straight, connected to RP by short crossvein r-m. Stem CuA straight, branching into CuA1 and CuA2 nearly at the same level of ending point of A1; CuA and CuA1 at 145° angle; CuA and CuA2 in alignment; CuA1 connected to M by short crossvein m-cua, at base of crossvein r-m. CuP strongly curved at the same level of ending point of A2.
Specimen STMN48-1808 (Fig. 3.3, 3.4): body with ovipositor as preserved 15.2 mm long. Head destroyed. Legs poorly preserved, obscure. Abdomen with ovipositor ~9.8 mm long and ~4.8 mm wide, with seven segments visible. Ovipositor extending just beyond forewing tips.
Forewings slender, ~13.3 mm long, 4.1 mm wide, with length/width ratio ~3.2. Basal portion of forewings deformed, basal portion of left forewing partly missing. Costal margin slightly arched. Posterior margin almost straight. Apical margin rounded. Costal area long and narrow; costal area of right forewing punctate at basal part, but not visible for left one. Clavus strongly deformed. Apices of costal area and clavus almost at the same level. Apical cells six. Pc+CP long and thicken, almost parallel to costal margin. ScP weak and short, running to and fusing with ScP+R+M+CuA, separating from the latter and then ending at apex of costal area. ScP+R+M+CuA bifurcating into ScP+R+M and CuA at junction with crossvein cua-cup; stem ScP+R+M extremely short. RA and RP unbranched, connected to each other by crossvein ir; RA slightly arched. Stem M two-branched; M1+2 and M3+4 connected to each other by crossvein im. Cell C3 ~1.5 times as long as adjoining apical cell. Stem CuA branching into CuA1 and CuA2 just basad of apices of costal area and clavus. CuP, A1, and A2 obscure.
Hind wing slightly shorter than forewing, with distal portion obviously darkly stained, without peripheric membrane. RA and RP nearly straight. Vein M unbranched, nearly straight, connected to RP by short crossvein r-m. Stem CuA straight; CuA and CuA1 at 145° angle; CuA and CuA2 in alignment; CuA1 connected to M by short crossvein m-cua, at base of crossvein r-m; CuA1 slightly longer than CuA2. Veins CuP, A1, and A2 obscure.
Remarks
The two new specimens are attributed to the genus Jiania Wang and Szwedo in Wang et al., Reference Wang, Szwedo and Zhang2012, based on the following diagnostic characters of the forewing: apical margin rounded, ScP+R+M+CuA bifurcating into ScP+R+M and CuA near basal one-fifth wing length, stalk M+R present, and bifurcation of M distad of ending point of A1 and basad of bifurcation of CuA. The new specimens are assigned to Jiania gracila Wang and Szwedo in Wang et al., Reference Wang, Szwedo and Zhang2012 based on their long ovipositor extending slightly beyond forewing tips and forewing with cell C3 ~1.5 times as long as adjoining apical cell (versus ovipositor distinctly exceeding tip of forewing and forewing with cell C3 almost as long as adjoining apical cell in Jiania crebra Wang and Szwedo in Wang et al., Reference Wang, Szwedo and Zhang2012).
Although most morphological characters of the body structures and forewings are similar, the two specimens described herein are different from the holotype in the size, length/width ratio, and surface ornament of the costal area and clavus of the forewing. Recently, some studies based on abundant specimens demonstrated that the obvious variation in size of body and wings and length/width ratio of forewing occurs intraspecifically in fossil froghoppers (Wang and Zhang, Reference Wang and Zhang2009; Wang et al., Reference Wang, Szwedo and Zhang2012; Li et al., Reference Li, Shih, Wang, Pang and Ren2013; Chen et al., Reference Chen, Wang, Zhang, Wang and Zheng2015a, Reference Chen, Zhang, Wang, Zheng and Wangb). Therefore, it is reasonable to consider the variation between the holotype and the two new specimens to be intraspecific. Forewing with clavus and basal costal area punctate is treated as important diagnostic character for Jiania gracila Wang and Szwedo in Wang et al., Reference Wang, Szwedo and Zhang2012 (Wang et al., Reference Wang, Szwedo and Zhang2012). In the new specimen STMN48-1809, both forewings possess costal areas that are entirely punctate, but clavus with puncta invisible. In STMN48-1808, puncta are only preserved on basal part of costal area of right wing. Tiny puncta are also poorly preserved or even completely missing for some sinoalid specimens reported in Wang et al. (Reference Wang, Szwedo and Zhang2012). The location of puncta on the forewing might be variable for different sinoalids, but considering taphonomical and preserving factors, it is imprudent to identify taxa based on this single character.
Jiania sp.
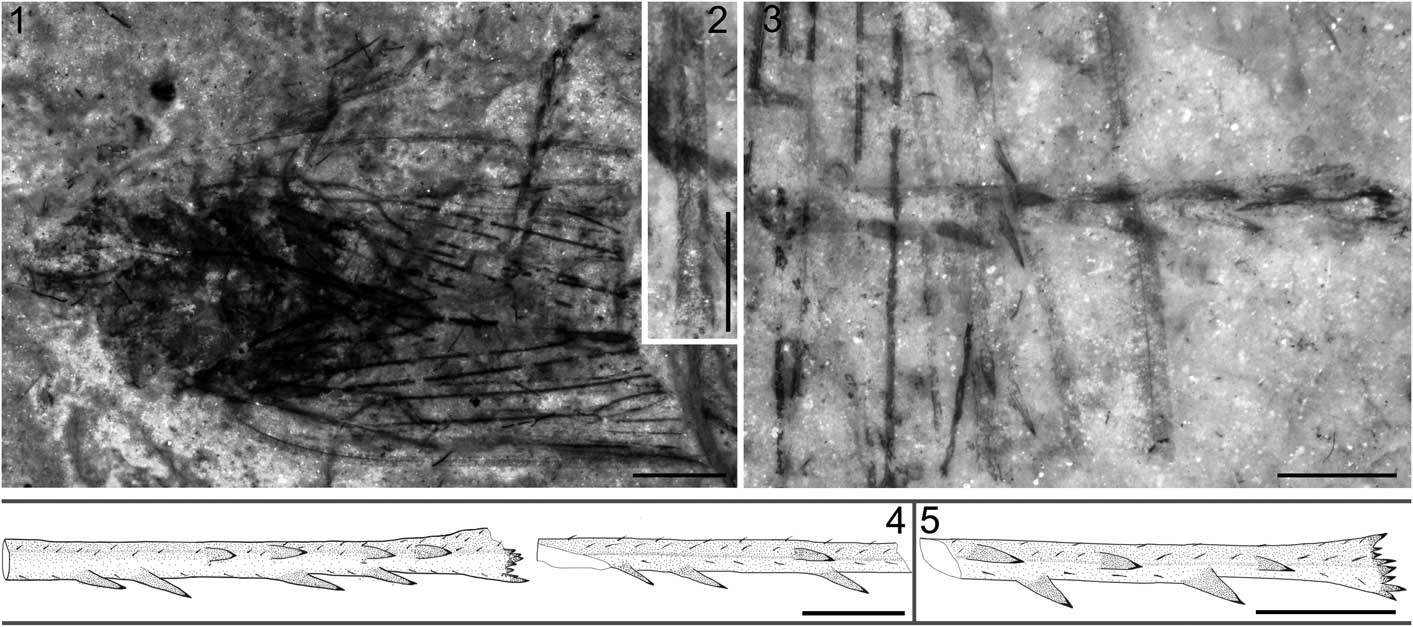
Figure 4 Jiania sp. STMN48-1810 and reconstructions of hind tibiae of Jiania Wang and Szwedo in Wang et al., Reference Wang, Szwedo and Zhang2012: (1) Jiania sp. STMN48-1810, under alcohol; (2) enlarged fore tibiae of Jiania sp. STMN48-1810, under alcohol; (3) enlarged hind tibiae of Jiania sp. STMN48-1810, under alcohol; (4) reconstructions of hind tibiae of Jiania sp. STMN48-1810; (5) reconstruction of hind tibia of Jiania crebra Wang and Szwedo in Wang et al., Reference Wang, Szwedo and Zhang2012, specimen NIGP154599. Scale=2 mm (1) or 1 mm (2–5).
Materials
STMN48-1810, sex unknown, adult in dorsoventral aspect with wings preserved at the top of the body, middle and distal portion of wings destroyed.
Occurrence
This specimen was collected from Daohugou Village (41°18′N, 119°13′E), Shantou Township, Ningcheng County of Inner Mongolia. The fossil bed is Callovian–Oxfordian (latest Middle–earliest Late Jurassic) in age.
Description
Specimen STMN48-1810 (Fig. 4.1–4.4): head partly preserved. Compound eyes missing. Rostrum extending to hind coxae. Antennae length as preserved ~0.6 mm; flagellum filiform, with segments invisible. Pronotum partly destroyed, ~1.7 mm long. Mesonotum ~3.1 mm long in midline and ~3.5 mm wide at base. Fore femur strong, basal part invisible, ~0.6 mm wide; fore tibia ~2.5 mm long, setose, with distinct ridges; fore tarsus ~1.4 mm long; basitarsomere very short; apical tarsomere slightly longer than mid tarsomere; two claws visible. One middle leg preserved; middle femur slightly slenderer than fore femur; middle tibia shorter than fore tibia, setose, with distinct ridges; middle tarsus obscure; tarsal claws distinct. One hind leg nearly completely preserved, other one with tarsus and apical part of tibia missing; hind femur nearly as long as fore femur; hind tibia slender, ~3.7 mm long, with two rows of lateral spines (each row with four spines for the complete tibia, but only one and three for the tibia with apical part missing) and a row of apical teeth; basitarsomere as long as mid tarsomere, apical tarsomere and claws destroyed. Abdomen invisible.
Forewings with middle and distal part missing, length as preserved 8.9 mm; one forewing obviously deformed. Costal margin slightly arched. Posterior margin as preserved almost straight. Costal area and clavus arched, long and narrow. Pc+CP long, almost parallel to costal margin. ScP weak and short, running to and fusing with ScP+R+M+CuA. ScP+R+M+CuA, bifurcating into ScP+R+M and CuA nearly at junction with crossvein cua-cup; stem ScP+R+M extremely short. Stem M nearly straight as preserved; branching into M1+2 and M3+4 basad of bifurcation of CuA and distad of ending point of A1. Stem CuA strongly curved at base, and then nearly straight as preserved. CuP strongly curved at junction with crossvein cua-cup, and then nearly straight. A1 nearly straight and A2 slightly sinuous. Hind wing obscure.
Remarks
This specimen undoubtedly belongs to the family Sinoalidae based on its body structures and unique forewing with Pc+CP long, almost parallel to costal margin, ScP weak and short, and R, M and CuA divided from stem ScP+R+M+CuA very closely. We placed the new specimen in the genus Jiania Wang and Szwedo in Wang et al., Reference Wang, Szwedo and Zhang2012 based on the following venational characters of forewings: short stalk ScP+R+M existing (vs. short stalk M+CuA existing in Sinoala Wang and Szwedo, 2012 and R, M, CuA separated from stem ScP+R+M+CuA at the same point in Luanpingia Hong, Reference Hong1983 and Huabeicercopis Hong, Reference Hong1983) and M branching into M1+2 and M3+4 basad of bifurcation of CuA and distad of ending point of A1 (versus M branching into M1+2 and M3+4 basad of ending point of A1 in Sinoala Wang and Szwedo, 2012 and M branching into M1+2 and M3+4 just distad of bifurcation of CuA in Shufania n. gen.). However, due to incomplete preservation and somewhat deformation of the specimen, it is impossible to get more specific characters to compare it with the other two congeneric species in detail. Therefore, we herein maintain the specimen in open nomenclature in the genus Jiania Wang and Szwedo, 2012.
Discussion
The Sinoalidae, with six known genera, is known so far only from the Middle to earliest Late Jurassic of northeastern China (Wang et al., Reference Wang, Szwedo and Zhang2012; this study). The new genus reported in the present study is distinct from all other genera in forewing venations, leading to the necessity to revise the family Sinoalidae. Bifurcation of vein M basad of bifurcation of CuA on forewing is considered as one of the important diagnostic characters for the Sinoalidae, differing from the con-superfamilial Procercopidae. However, Shufania n. gen. possesses a forewing with bifurcation of vein M apparently distad of bifurcation of CuA. Chen et al. (Reference Chen, Zhang, Wang, Zheng and Wang2015b) erected a new species, Anthoscytina elegans, based on ten well-preserved procercopid fossils collected from the Daohugou Biota. These specimens show high intraspecific variation on relative branching position of M and CuA. Therefore, this venational character, unstable even at species level for the Cercopoidea, is not appropriate for family-level diagnosis.
Up to now, only two sinoalid species with information on hind wings have been reported (Hong, Reference Hong1983; Wang et al., Reference Wang, Szwedo and Zhang2012). Wang et al. (Reference Wang, Szwedo and Zhang2012) established Sinoala parallelivena based on several fossils from the latest Middle-earliest Late Jurassic of Inner Mongolia, China, including information of body structures, forewings, and hind wings. Hong (Reference Hong1983) erected Hebeicercopis triangulata based on an almost completely isolated hind wing and Huabeicercopis yangi mainly based on forewings from the Middle Jurassic of Heibei, China. Wang et al. (Reference Wang, Szwedo and Zhang2012) considered that these two taxa might be synonymous because of the corresponding size and the same original horizon and locality. We herein report two new imprint fossils of Jiania gracila with some interesting information about sinoalid hind wings. Although the forewings of the genera Huabeicercopis, Sinoala, and Jiania are significantly different (see Wang et al., Reference Wang, Szwedo and Zhang2012), the venations of the three sinoalid hind wings are obviously less variable, which is expected because the venations of sinoalid hind wings are very simplified in topology and obviously reduced in branches of longitudinal veins.
Hind wings with distal portion obviously darkly stained are well preserved for STMN48-1808, and visible for STMN48-1809 and most of the reported Jiania fossils (Fig. 3; Wang et al., Reference Wang, Szwedo and Zhang2012). However, this color pattern is completely absent for the seven well-preserved Sinoala fossils in Wang et al. (Reference Wang, Szwedo and Zhang2012), so hind wings of this genus are likely colorless and transparent. Color patterns of hind wings are likely variable for different sinoalid froghoppers. However, wing color pattern is easily weakened or even erased by diagenetic processes for imprint fossils, so the morphological character is not reliable to distinguish sinoalids, as is the case for other fossil cicadomorphs (e.g., Chen et al., Reference Chen, Zhang, Wang, Zheng, Wang and Zheng2016).
Modern taxa of the Cercopoidea possess hind tibia with one row of immobile spines (1–6, commonly 2) (Wang et al., Reference Wang, Szwedo and Zhang2012). Some studies based on abundant whole-body fossils suggest that the Procercopidae (Shcherbakov and Popov, Reference Shcherbakov and Popov2002) likely just has one single spine (Shcherbakov, Reference Shcherbakov1988; Wang and Zhang, Reference Wang and Zhang2009; Li et al., Reference Li, Shih, Wang, Pang and Ren2013; D. Chen et al., Reference Chen, Yao and Ren2015; Chen et al., Reference Chen, Wang, Zhang, Wang and Zheng2015a). The Sinoalidae differs from the Procercopidae and modern Cercopoidea in having hind tibia with two rows of spines laterally (Fig. 4.4, 4.5; at most four for each row, as shown in the new specimen STMN48-1810). The left and right hind tibiae preserved in STMN48-1810 suggest that lateral spines might be different in number and/or position (Fig. 4.4). Lateral spines (4–6 in number) on the hind tibia of the living aphrophorid Sinophora Melichar, 1902 (Anufriev, Reference Anufriev1972; Liang, Reference Liang1990) are sometimes variable in number for the same species or even the same individuals (Chou et al., Reference Chou, Yuan and Liang1986; Wang et al., Reference Wang, Szwedo and Zhang2012). STMN48-1810 indicates that the number of spines of the hind tibia might be also different intra-individually within the Sinoalidae, or at least their relative position is variable.
Acknowledgments
The authors are extremely grateful to B. Wang and H. Zhang for their constructive comments on an earlier version of the manuscript. The present study was supported by grants from the National Natural Science Foundation of China (41502007), the Natural Scientific Foundation of Shandong Province (ZR2013DQ017), and China Postdoctoral Science Foundation (2015M580480). Many thanks go to two editors, J. Jin and J. Hannibal, and two reviewers, A. Nel and J. Szwedo, who provided many constructive comments, which undoubtedly improved this manuscript.






