Introduction
Classification of the order Redlichiida Richter, Reference Richter, Dittler, Joos, Korschelt, Linek, Oltmanns and Schaum1932 and its main representative groups is an old controversy (e.g., Richter, Reference Richter, Dittler, Joos, Korschelt, Linek, Oltmanns and Schaum1932; Harrington, Reference Harrington and Moore1959; Bergström, Reference Bergström1973) that found a satisfying, but not consensual, solution through Palmer and Repina's (Reference Palmer and Repina1993) proposal. Two suborders—Olenellina Walcott, Reference Walcott1890 and Redlichiina Richter, Reference Richter, Dittler, Joos, Korschelt, Linek, Oltmanns and Schaum1932—are recognized, the most conspicuous character separating the two being the lack of facial sutures in the former. Apart from this character, the phylogenetic value of which is arguable (see Jell, Reference Jell2003), olenellines and redlichiines are morphologically very similar, including in their developmental stages (Whittington, Reference Whittington1989; Briggs and Fortey, Reference Briggs, Fortey, Lipps and Signor1992). Both Olenellina and Redlichiina are widely considered paraphyletic (e.g., Geyer, Reference Geyer1996; Fortey, Reference Fortey and Kaesler1997; Adrain, Reference Adrain2011).
Olenellina is considered the most primitive group in the Trilobita, being characteristic of the late early Cambrian of Laurentia (although some olenellids are now equivalent in age to the basal paradoxidids; Sundberg et al., Reference Sundberg, Geyer, Kruse, McCollum, Pegel, Zylinska and Zhuravlev2016, Reference Sundberg, Karlstrom, Geyer, Foster, Hagadorn, Mohr, Schmitz, Dehler and Crossey2020) and a minor component of the trilobite faunas of Siberia, Baltica, Avalonia, and West Gondwana (Palmer and Repina, Reference Palmer and Repina1993). Curiously, olenellines are hitherto unknown from coeval sequences of eastern Gondwana. This suborder established the base of the lower Cambrian biostratigraphic subdivisions (see Palmer and Repina, Reference Palmer and Repina1993, Reference Palmer, Repina and Moore1997) and early Cambrian biogeography (Pillola, Reference Pillola1991; McKerrow et al., Reference McKerrow, Scotese and Brasier1992). In addition, Olenellina has provided outstanding information about evolutionary trends (e.g., Fortey et al., Reference Fortey, Briggs and Wills1996; Smith and Lieberman, Reference Smith and Lieberman1999; Lieberman, Reference Lieberman2002; Paterson and Edgecombe, Reference Paterson and Edgecombe2006; Paterson et al., Reference Paterson, Edgecombe and Lee2019) and adaptive strategies of the basal trilobite faunas (e.g., Ortega-Hernández et al., Reference Ortega-Hernández, Esteve and Butterfield2013).
Classification within Olenellina has also been historically controversial (see Palmer and Repina, Reference Palmer and Repina1993) and it is still problematic. The revision carried out by those authors and adopted in the Treatise on Invertebrate Paleontology (Palmer and Repina, Reference Palmer, Repina and Moore1997) considered two superfamilies: Olenelloidea Walcott, Reference Walcott1890 and Fallotaspidoidea Hupé, Reference Hupé1953. Considerably different from previous schemes (e.g., Bergström, Reference Bergström1973; Ahlberg et al., Reference Ahlberg, Bergström and Johansson1986), this classification was soon questioned by Geyer (Reference Geyer1996) and modified by Lieberman (Reference Lieberman1998, Reference Lieberman1999, Reference Lieberman2001), who divided Olenellina into three superfamilies (Olenelloidea, Judomioidea Repina, Reference Repina1979, and Nevadioidea Hupé, Reference Hupé1953) and removed Fallotaspidoidea based on a phylogenetic analysis. Later works (e.g., Webster, Reference Webster2007; Webster and Bohach, Reference Webster and Bohach2014; Webster and Landing, Reference Webster and Landing2016) pointed out several coding errors in these analyses and criticized the methodology (e.g., absence of ontogenetic studies). A comprehensive cladistic analysis of Olenellina is currently in preparation by M. Webster (Webster and Hageman, Reference Webster and Hageman2018).
Olenelline trilobites are very rare in the lower Cambrian of the Iberian Peninsula and thus are poorly studied. Presently, only three species are described: Paradoxides choffati Delgado, Reference Delgado1904 from Vila Boim (Elvas, Portugal), Callavia? lotzei Richter and Richter, Reference Richter and Richter1941 from Cañaveral de León (Huelva, Spain), and Andalusiana cornuta Sdzuy, Reference Sdzuy1961 from Guadalcanal (Seville, Spain). Paradoxides choffati was later transferred to Callavia Matthew, Reference Matthew1897 by Richter and Richter (Reference Richter and Richter1941) and Teixeira (Reference Teixeira1952). In contrast, Sdzuy (Reference Sdzuy1962) established several similarities between Callavia? lotzei and the genus Judomia Lermontova, Reference Lermontova1951 and Sdzuy (Reference Sdzuy2001) transferred the former to the latter. Finally, Lieberman (Reference Lieberman2001) used Callavia? lotzei to erect the new genus Sdzuyomia Lieberman, Reference Lieberman2001.
In the present paper, Callavia? lotzei and Paradoxides choffati are revised based on previously studied and new specimens from the Cumbres de San Bartolomé, Cañaveral de León, Sierra del Bujo, and Hinojales fossil sites and the type material from Vila Boim, respectively, to clarify their taxonomy, biostratigraphy, and paleobiogeography. The new data suggest that these taxa are conspecific and support the assignment of the Iberian species to Callavia. As a result, Sdzuyomia is herein considered a junior synonym of Callavia. The biogeographical implications of the new systematic data are discussed in the context of early Cambrian paleogeography.
Geological setting
The studied material came from the Vila Boim locality in the Elvas Municipality, southwestern Portugal (type locality of Paradoxides choffati), and from four municipalities in northern Huelva province, southwestern Spain: Cumbres de San Bartolomé, Cañaveral de León (type locality of Callavia? lotzei), Arroyomolinos de León (Sierra de El Bujo), and Hinojales. These fossil localities are located in the Ossa-Morena Zone in the southern branch of the Iberian Massif, which comprises a lithostratigraphic sequence ranging in age from terminal Proterozoic to the Carboniferous, with a general structure of large, recumbent folds verging to the southwest (Azor, Reference Azor and Vera Torres2004). Cambrian outcrops of the Ossa-Morena Zone are divided into tectonosedimentary units classically called sectors in Portugal (Oliveira et al., Reference Oliveira, Oliveira and Piçarra1991) and ‘Cubetas’ in Spain, which correspond to ancient sedimentary basins limited and controlled by faults according to Liñán and Quesada (Reference Liñán, Quesada, Dallmeyer and Martínez García1990). The Portuguese locality is located in the Alter do Chão-Elvas sector and the Spanish outcrops in the Cumbres and Herrerías ‘Cubetas,’ representing the southwesternmost fossiliferous units in lower Cambrian times for Iberia (Fig. 1.1).

Figure 1. (1) Pre-Hercynian outcrops in the Iberian Peninsula. (2) Geological setting of fossil sites in the Cambrian sectors (fault-bounded blocks) of the Ossa-Morena Zone, indicating the position of the studied fossil sites (modified from Liñán and Quesada, Reference Liñán, Quesada, Dallmeyer and Martínez García1990). (3) Stratigraphic column of the Alter do Chão–Elvas sector (modified from Liñán et al., Reference Liñán, Perejón, Gozalo, Moreno-Eiris and Oliveira2004). (4) Stratigraphic column of the Cumbres ‘cubeta’ (after Collantes et al., Reference Collantes, Mayoral, Chirivella and Gozalo2020). (5) Stratigraphic column of the Herrerías ‘cubeta’ (after Collantes et al., Reference Collantes, Mayoral, Chirivella and Gozalo2020).
The Vila Boim fossil site is a classical outcrop at Monte Valbom (Fig. 1.2), first published by Delgado (Reference Delgado1904) and located in the lower part of the Vila Boim Formation, a 600 m thick succession composed of shales, quartzites, and graywackes with some interbedded rhyolite and basalt levels (Mata, Reference Mata1986) (Fig. 1.3). All specimens originate from a narrow lenticular horizon of blue-gray shales with iron oxides, bearing two 15 cm thick fossiliferous levels separated by 1 m, located ~170 m from the base of the Vila Boim Formation. Callavia choffati is rare in the fossil assemblage, which is dominated by the trilobites Hicksia elvensis Delgado, Reference Delgado1904 and Delgadella souzai (Delgado, Reference Delgado1904), with fewer brachiopods, hyolithids, and bivalves (see Delgado, Reference Delgado1904; Teixeira, Reference Teixeira1952). According to Liñán et al. (Reference Liñán, Perejón, Gozalo, Moreno-Eiris and Oliveira2004), this fossil assemblage suggests a middle Marianian age (ca. 515 Ma). The fossiliferous section is located at 38°52′2″N, 007°17′31″W.
The fossil levels from the Cumbres de San Bartolomé section belong to the ‘Alternancia de Cumbres’ (Cumbres beds), an informal lithostratigraphic unit composed of a succession of shale and sandstone of very variable thickness (350−1,100 m). The specimens studied herein were recorded from the lower levels (Fig. 1.4), together with the trilobites Atops calanus Richter and Richter, Reference Richter and Richter1941 and Delgadella souzai, considered middle Marianian in age. A second level, 400 m above the former, contained a more diverse upper Marianian trilobite assemblage (Collantes et al., Reference Collantes, Mayoral, Liñán and Gozalo2021). The base and top of the section are located at 38°03′16″N, 006°43′00″W and 38°03′14″N, 006°42′58″W, respectively.
The sections outcropping in Sierra del Bujo (Richter and Richter, Reference Richter and Richter1941), Hinojales (Liñán and Mergl, Reference Liñán and Mergl1982), and the Cañaveral de León fossil sites represent equivalent stratigraphic levels assigned to the unit ‘Pizarras de Herrerías’ (Herrerías shale), characterized by purple shales with spilitic intercalations within a sequence 200–600 m thick (Fig. 1.5). This unit ranges from the middle to upper Marianian, indicated by the presence of trilobites Rinconia-Ellipsostrenua in the lower levels and Serrodiscus-Triangulaspis in the upper levels. Sierra del Bujo section is located between 38°00′39″N, 006°27′34″W and 38°00′39″N, 006°27′38″W; Hinojales section is located between 38°00′26″N, 006°35′08″W and 38°00′24″N, 006°35′06″W; and Cañaveral de León section is located between 38°01′05″N, 006°32′23″W and 38°01′02″N, 006°32′28″W.
Materials and methods
Available material consists mainly of isolated cephala preserved as internal or external molds, with isolated pygidia and one mostly complete, articulated exoskeleton. Specimens preserved in shales are often flattened and distorted, whereas those preserved in sandstones retain some original convexity. Specimens from Portugal were collected by the end of the nineteenth century and previously figured by Delgado (Reference Delgado1904) and Teixeira (Reference Teixeira1952), whereas samples from Huelva, Spain, were collected in several campaigns from 1985 to the present by the authors; these have intensified since 2018.
Specimens were prepared using a pneumatic hammer, coated with ammonium chloride, and photographed using a Canon EOS 77D coupled with a Canon 100 mm f/2.8L macro lens. Terminology follows that of the revised Treatise on Invertebrate Paleontology (Palmer and Repina, Reference Palmer, Repina and Moore1997).
Abbreviations used in the text are: exsag. = exsaggital; L1, L2, etc. = glabellar lobes; LA = frontal lobe; LO = occipital lobe; S1, S2, etc. = glabellar furrows; sag. = sagittal; SO = occipital furrow; tr. = transversa; v = specimens have been visited in their collection and seen in person; * = type species.
Repositories and institutional abbreviations
Figured specimens are housed in the Department of Earth Sciences (Laboratory of Tectonics and Paleontology) of the Faculty of Experimental Sciences, University of Huelva, Spain (UHU) and in the Museu Geológico de Lisboa, Lisbon, Portugal (MG).
Systematic paleontology
Class Trilobita Walch, Reference Walch1771
Order Redlichiida Richter, Reference Richter, Dittler, Joos, Korschelt, Linek, Oltmanns and Schaum1932
Suborder Olenellina Walcott, Reference Walcott1890
Superfamily ‘Judomioidea’ Repina, Reference Repina1979 (sensu Lieberman, Reference Lieberman2001)
Remarks
The systematic position of Callavia has been controversial. Although most authors have nested it with members of Holmiidae Hupé, Reference Hupé1953 (e.g., Harrington, Reference Harrington and Moore1959; Chernysheva, Reference Chernysheva1960; Repina, Reference Repina1979), Bergström (Reference Bergström1973) included Callaviinae Poulsen in Harrington, Reference Harrington and Moore1959 in Daguinaspididae Hupé, Reference Hupé1953 and Ahlberg et al. (Reference Ahlberg, Bergström and Johansson1986) preferred to treat it as an independent family, not related to holmiinids. Following the most consensual assignment, Palmer and Repina (Reference Palmer and Repina1993, Reference Palmer, Repina and Moore1997) maintained Callaviinae within Holmiidae, thus including Callavia within the superfamily Olenelloidea. Nevertheless, they assigned the family Judomiidae, morphologically similar to callaviinines, to the superfamily Fallotaspidoidea, revealing an inadequacy of this proposal for these taxa. In fact, following the concept of Palmer and Repina (Reference Palmer and Repina1993), Callavia cannot be included within Olenelloidea, and, consequently, in Holmiidae, because it lacks some of the diagnostic characters of the superfamily (frontal lobe [LA] enlarged and ocular lobe connected only to posterolateral part of LA, both absent in Callavia) and of the family (extraocular area [tr.] wider than twice the width of the interocular area, unlike in all holmiids). This was also stated by Lieberman (Reference Lieberman1998, Reference Lieberman1999, Reference Lieberman2001), who treated Callavia and a group of ‘fallotaspidoids’ (sensu Palmer and Repina, Reference Palmer and Repina1993) as representing an independent taxon of superfamiliar rank, the Judomioidea (not Nevadioidea, as mistakenly considered by Fletcher and Theokritoff, Reference Fletcher and Theokritoff2008).
One of the Callavia morphological characters that was misinterpreted by Palmer and Repina (Reference Palmer and Repina1993) and that were used to justify previous assignments to Olenelloidea/Holmiidae, is the relation between the ocular lobe and the LA. This relationship was considered the principal phylogenetic trend within the Olenellina by Palmer and Repina (Reference Palmer and Repina1993), with the earliest representatives having a glabella that is parallel-sided or tapering forward and an ocular lobe that is attached along the entire margin of the LA. Callavia shows this condition (see remarks on the genus). Nevertheless, it has been previously described as though the LA becomes inflated and expanded laterally and the ocular lobes connect only to its posterior part (like in Olenellidae and Holmiidae). Despite several errors in Lieberman's (Reference Lieberman1998, Reference Lieberman1999, Reference Lieberman2001) phylogenetic analyses (e.g., Webster, Reference Webster2007, Reference Webster2009), and the very limited and unrepresentative number of species coded, we herein prefer to assign Callavia to the (questionably monophyletic) ‘Judomioidea’ (sensu Lieberman, Reference Lieberman2001) instead of Olenelloidea, and we avoid family assignment within it.
Genus Callavia Matthew, Reference Matthew1897
Type species
Olenellus (Mesonacis) broeggeri Walcott, Reference Walcott1890 from the Brigus Formation, Branchian Series (Cambrian Stage 3/4), Newfoundland, Canada.
Other species
Olenellus (Holmia) callavei Lapworth, Reference Lapworth1891 from the Comley Limestone Formation, Branchian Series (Cambrian Series 2), Shropshire, UK; Paradoxides choffati Delgado, Reference Delgado1904 from the lower part of the Vila Boim Formation, Marianian (Cambrian Series 2), Vila Boim, Portugal (see Table 1).
Table 1. List of taxa previously assigned to Callavia Matthew, Reference Matthew1897 and their currently accepted generic assignment. Notes: 1—Fletcher and Theorokritoff (Reference Fletcher and Theokritoff2008) regarded Callavia broeggeri (Walcott, Reference Walcott1890) and Callavia crosbyi (Walcott, Reference Walcott1890) as different species; 2—Lieberman (Reference Lieberman1999) considered Paedeumias Walcott, Reference Walcott1910 a junior synonym of Olenellus Hall, Reference Hall1862. Webster (personal communication, 2021) regarded Paedeumias breviloba Poulsen, Reference Poulsen1927 as an indeterminate olenelline species.

Emended diagnosis
Posterior margin and posterior furrow of cephalon curved forward; base of genal spine lying slightly posterior to lateral margins of LO; genal spine broad-based; intergenal spine present, prominent to reduced; cephalic border developed as rounded ridge; anterior and lateral border furrows broad and deep; long tropidium-like structure extending across the lateral and anterior border furrows. Glabella subcylindrical, slightly tapered anteriorly; LA not contacting anterior border furrow, surrounded by a weak parafrontal band; LA not enlarged; preglabellar field very short, almost indistinct; occipital furrow (SO) not conjoined medially; occipital spine present; four preoccipital glabellar furrows (L1−L4) shallowing anteriorly, nontransglabellar, straight to slightly obliquely backward, when followed adaxially. Ocular lobe prominent; inner margin differentiated from a broad interocular area; extraocular area slightly narrower to slightly wider (tr.) than interocular area opposite S1; posterior tip of ocular lobe opposite SO. Intergenal ridge and posterior ocular line subparallel to converging toward the intergenal spine/swelling. Sculpture of reticulated pattern on external surface and terrace ridges along the abaxial limit of the anterior border.
Remarks
Callavia is one of those genera for which previous documentation and assigned species strongly exceed its currently accepted diversity (Table 1). In its most recent concepts, Callavia is extremely poorly diverse, ranging from monotypic (Lieberman, Reference Lieberman2001) to including only two species (e.g., Landing et al., Reference Landing, Westrop and Bowring2013b). Although describing Callavia as “the principal genus of the Olenellina from Avalonia,” Palmer and Repina (Reference Palmer and Repina1993, p. 14) considered a greater species diversity. This could also have led these authors to diagnose Callavia with characters that are not present in the type species, Callavia broeggeri. In fact, the figured material of this species is quite limited (Grabau, Reference Grabau1900; Walcott, Reference Walcott1910; Hutchinson, Reference Hutchinson1962; Landing et al., Reference Landing, Nowlan and Fletcher1980; Palmer and Repina, Reference Palmer and Repina1993; Lieberman, Reference Lieberman2001; Fletcher, Reference Fletcher2006), being mostly deformed or fragmented, including the type material (as stated by Hutchinson, Reference Hutchinson1962, p. 119).
In our opinion, several morphological characters have been misinterpreted: (1) presence or absence of the preglabellar field, (2) the tropidium-like ridge, and (3) the parafrontal band. Palmer and Repina (Reference Palmer and Repina1993) and Lieberman (Reference Lieberman2001) considered a preglabellar field as absent, with the frontal lobe directly contacting the anterior border furrow. Nevertheless, several illustrations of type and other material of Callavia broeggeri (and its possible junior synonym Callavia crosbyi Walcott, Reference Walcott1910) by Walcott (Reference Walcott1890, pl. 91, fig. 1, pl. 92, fig. 1, 1g; Reference Walcott1910, pl. 27, figs. 1, 4, pl. 28, fig. 4), as well as other figured specimens (e.g., Hutchinson, Reference Hutchinson1962, pl. 24, figs. 8–11; Palmer and Repina, Reference Palmer and Repina1993, fig. 6.8), clearly show a short but defined preglabellar field. One of the features that had contributed to this misinterpretation is the presence of a tropidium-like structure (e.g., Walcott, Reference Walcott1890, pl. 92, fig. 1b; Reference Walcott1910, pl. 28, figs. 1, 4; Hutchinson, Reference Hutchinson1962, pl. 24, figs. 7b, 8, 9; Palmer and Repina, Reference Palmer and Repina1993, fig. 6.8; Lieberman, Reference Lieberman2001, fig. 2.1) that is adaxial to the true anterior border furrow. Furthermore, and as previously stated by Fletcher and Theokritoff (Reference Fletcher and Theokritoff2008), a weak parafrontal band is present around the LA margins (e.g., Walcott, Reference Walcott1910, pl. 28, figs. 1, 4; Hutchinson, Reference Hutchinson1962, fig. 7a; Palmer and Repina, Reference Palmer and Repina1993, fig. 6.8), a character that led previous authors to consider the preglabellar field as absent in Callavia broeggeri. The parafrontal band is also observed in Callavia callavei (already stated by Lake, Reference Lake1937) and Callavia choffati (being clear only in better preserved specimens).
The parafrontal band was also described by Walcott (Reference Walcott1910), who erected the new species Callavia crosbyi based on this character, among others. We agree with Lieberman (Reference Lieberman2001) who considered Callavia crosbyi as a junior synonym of Callavia broeggeri. Fletcher and Theokritoff (Reference Fletcher and Theokritoff2008) argued that Callavia crosbyi is a valid species, differing from Callavia broeggeri in having a much narrower (tr.) extraocular area and a distinct pygidium and posteriormost thoracic segments. Nevertheless, the only Callavia crosbyi specimen preserving the thorax and the pygidium (Fletcher and Theokritoff, Reference Fletcher and Theokritoff2008, fig. 5.16) shows an extraocular area proportionally similar to that of Callavia broeggeri.
Another misinterpreted character of Callavia broeggeri is the morphology of S1, which Lieberman (Reference Lieberman2001) considered conjoined medially and different from the condition observed in Callavia callavei. Based on this difference, he erected the new monotypic genus Callavalonia Lieberman, Reference Lieberman2001 for this latter species. Although the glabellar segmentation of Callavia callavei and Callavia broeggeri present some differences, namely the glabellar furrows are apparently more deeply incised and the anteriormost furrows longer (tr.) in the former, it is not possible to assure that S1 is conjoined medially in Callavia broeggeri. Fletcher and Theokritoff (Reference Fletcher and Theokritoff2008) also considered this character to be unrecognizable. In the studied material of Callavia choffati, the collapse due to the flattening of the glabella in some specimens created an artifact, with S0 or S1 appearing conjoined medially (e.g., Fig. 2.1, 2.5). Nevertheless, in specimens preserving glabellar convexity, either in Callavia choffati (e.g., Fig. 3.5, 3.16) or in Callavia broeggeri (see Palmer and Repina, Reference Palmer and Repina1993, fig. 6.8), it is clear that they are not transglabellar.

Figure 2. Callavia choffati (Delgado, Reference Delgado1904), Vila Boim Formation, middle Marianian, Vila Boim, Portugal: (1) MG 15781, lectotype; (2) MG 15782a, paralectotype; (3, 4) MG sn, paralectotype (latex): (3) dorsal view; (4) detail of terrace lines of the lateral cephalic border; (5) MG 15786, paralectotype; (6) MG 16684 (latex). Scale bars = 2 mm (4); 10 mm (1–3, 5, 6).

Figure 3. Callavia choffati (Delgado, Reference Delgado1904), ‘Herrerías shale,’ middle Marianian, Cañaveral de León (1−13, 15−20) and ‘Cumbres beds,’ middle Marianian, Cumbres de San Bartolomé (14), Spain: (1, 2) UHU-CVL 01: (1) dorsal view; (2) lateral view; (3) UHU-CVL 03; (4) UHU-CVL 05; (5, 6) UHU-CVL 09: (5) lateral view; (6) dorsal view; (7) UHU-CU 1/1/1; (8, 9) UHU-CVL 12: (8) dorsal view; (9) lateral view; (10) UHU-CVL 10; (11) UHU-CVL 17 (latex); (12) UHU-CVL 20; (13) UHU-CVL 40; (14−16) UHU-CVL 32: (14) dorsal view; (15) frontal view; (16) dorsal view; (17) UHU-CVL 29; (18) UHU-CVL 42 (latex); (19) UHU-CVL 46; (20) UHU-CVL 48. Scale bars = 2 mm (4); 5 mm (6, 7, 11, 13–16, 18); 10 mm (1–3, 5, 8–10, 12, 17). Arrows in 7, 8, 18, and 20 indicate intergenal spines.
Lieberman (Reference Lieberman2001) also differentiated Callavalonia from Callavia by the relative width of the thoracic pleural furrow, which he considered to extend approximately two-thirds of the width of the inner pleural region in Callavia callavei, being longer (tr.; approximately four-fifths) in Callavia broeggeri. This character seems to be dependent on the thoracic segment number as well as on preservation. On a complete specimen of Callavia choffati (Fig. 4.1), it is possible to verify pleural furrows extending to different widths of the inner pleura (e.g., compare the first, second, third, and sixth segments). On the other hand, complete specimens of Callavia broeggeri (see Palmer and Repina, Reference Palmer and Repina1993, fig. 6.5) show a pleural furrow extension similar to that of Callavia callavei (occupying only two-thirds of the inner pleura). For these reasons, we agree with Jell and Adrain (Reference Jell and Adrain2002), Fletcher (Reference Fletcher2006), Fletcher and Theokritoff (Reference Fletcher and Theokritoff2008), and Landing et al. (Reference Landing, Westrop and Bowring2013b), who treated Callavalonia as a junior synonym of Callavia. Nevertheless, we do not concur with Fletcher (Reference Fletcher2006) and Fletcher and Theokritoff (Reference Fletcher and Theokritoff2008), who treated Callavia broeggeri and Callavia callavei as synonyms. Landing et al. (Reference Landing, Westrop and Bowring2013b) criticized this synonymy based on different eye-lobe positions, and we add cephalic segmentation to the list of differences between the two species.
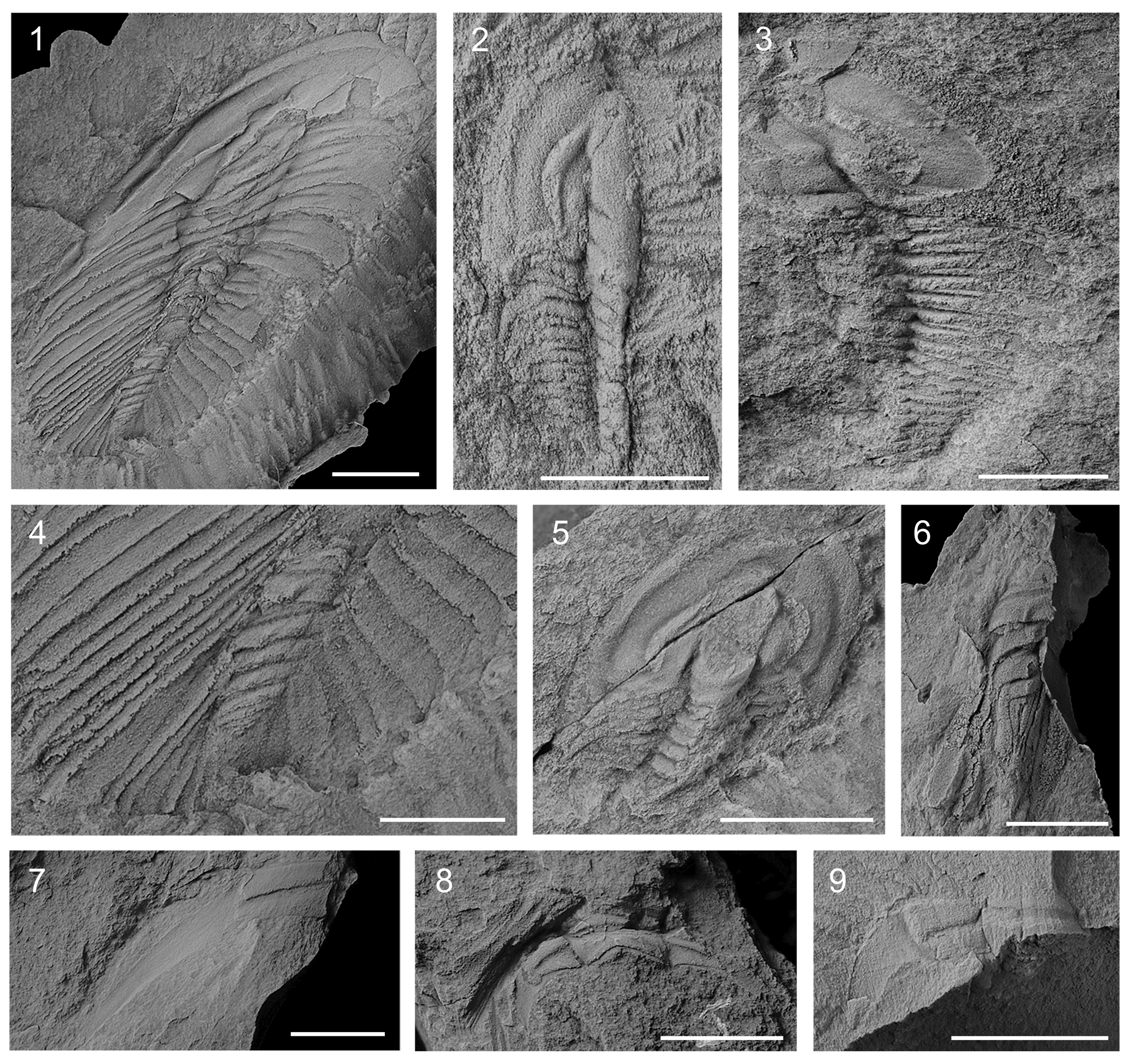
Figure 4. Callavia choffati (Delgado, Reference Delgado1904), Vila Boim Formation, middle Marianian, Vila Boim, Portugal (1, 4, 6–9) and ‘Herrerías Shale,’ middle Marianian, Cañaveral de León, Spain (2, 3, 5): (1, 4) MG 15787b, paralectotype (latex): (1) overview; (4) detail of pygidium; (2) UHU-CVL 49; (3) UHU-CVL 36; (5) UHU-CVL 37; (6) MG 15707b, paralectotype (latex); (7) MG 15764, paralectotype; (8) MG 15714, paralectotype; (9) MG 16658, paralectotype. Scale bars = 5 mm (2–5); 10 mm (1, 6–9).

Figure 5. Distribution of Callavia plotted on the Cambrian paleogeographic map (modified from Scotese and McKerrow, Reference Scotese and McKerrow1990; McKerrow et al., Reference McKerrow, Scotese and Brasier1992; Dalziel, Reference Dalziel1997; and Malinky and Geyer, Reference Malinky and Geyer2019).
Lieberman (Reference Lieberman2001) erected Sdzuyomia as a monotypic genus represented by Callavia? lotzei (herein revised). We consider Callavia? lotzei a junior synonym of Paradoxides choffati (see remarks on species) and assign it to the genus Callavia. Consequently, Sdzuyomia is treated as a junior synonym of Callavia. Lieberman (Reference Lieberman2001) carried out a phylogenetic analysis of the suborder Olenellina and erected the genus Sdzuyomia to incorporate solely the type species Callavia? lotzei, grouping it within the superfamily Judomioidea with Callavalonia (= Callavia), Bondonella Hupé, Reference Hupé1953, Neltneria Hupé, Reference Hupé1953, Callavia, and Judomia. Based on the new specimens from the Cumbres de San Bartolomé, Cañaveral de León, Sierra del Bujo, and Hinojales fossil sites, it is possible to verify that Lieberman's (Reference Lieberman2001) erection of Sdzuyomia was based on miscoded characters in both the Spanish species and Callavia broeggeri. Among other characters, he considered the intergenal spines as absent in Sdzuyomia (character 39) and that in Callavia, the LA contacts the anterior furrow (character 5), the S1 is conjoined medially (character 30), and the thoracic pleural furrows occupy almost all of the inner pleural region (character 51). In our opinion, these characters were misinterpreted by both Lieberman (Reference Lieberman2001) and Palmer and Repina (Reference Palmer and Repina1993). We do consider Callavia broeggeri, Callavia callavei, and Callavia choffati to be comparable in most of the significant olenelline features (cephalic outline, border structure, position and configuration of the ocular lobes and their relationship with the glabella, presence and position of the intergenal spines, glabellar outline and lobation, and thoracic structure). As Geyer (Reference Geyer2007) argued, the establishment of Sdzuyomia by Lieberman (Reference Lieberman2001) was premature, especially because to that date the known material of ‘Callavia? lotzei’ was poorly preserved and very limited. Currently, and with better knowledge of this Iberian species, we reinforce its assignment to Callavia.
Previous generic assignment of Spanish material (‘Callavia? lotzei’ = Callavia choffati) to the genus Judomia by Sdzuy (Reference Sdzuy2001) is here rejected. After comparison with figured material from Siberia (Khomentovskii and Repina, Reference Khomentovskii and Repina1965; Repina et al., Reference Repina, Lazarenko, Meshkova, Korshunov, Nikiforov and Aksarina1974; Korobov, Reference Korobov1989; Pegel, Reference Pegel2000; Ponomarenko, Reference Ponomarenko2005; Rozanov and Varlamov, Reference Rozanov and Varlamov2008) and Laurentia (Fritz, Reference Fritz1973; McMenamin, Reference McMenamin1987; Gapp et al., Reference Gapp, Lieberman, Pope and Dilliard2011), we do not agree that Callavia choffati mainly differs from Judomia in ocular structure. The ocular lobes in Judomia are located very close to the glabella, thus the interocular area is almost absent. Furthermore, the inner margin of the ocular lobe is undifferentiated or only weakly differentiated from the interocular area in Judomia, which is a very narrow (tr.), depressed area (e.g., J. granulata Repina in Repina et al., Reference Repina, Lazarenko, Meshkova, Korshunov, Nikiforov and Aksarina1974, J. mattajensis Lazarenko, Reference Lazarenko1962, J. tera Lazarenko in Kryskov et al., Reference Kryskov, Lazarenko, Ogienko and Tchernysheva1960, and J. rasskasovae Korobov, Reference Korobov1963 as figured by Repina et al., Reference Repina, Lazarenko, Meshkova, Korshunov, Nikiforov and Aksarina1974, pls. 27, 28). On the contrary, in Callavia, the interocular area is broad and inflated, almost the same width as the extraocular area opposite S1, and the inner margin of the ocular lobes is well differentiated from it. Other characters are the straight and deep posterior border furrow in Judomia (e.g., J. tera by Repina et al., Reference Repina, Lazarenko, Meshkova, Korshunov, Nikiforov and Aksarina1974, pl. 28, fig. 1; Palmer and Repina, Reference Palmer and Repina1993, fig. 10.7), being faint and curved forward in Callavia due to a prominent intergenal ridge; and the posteriormost LA in direct contact with the ocular lobes in Judomia (e.g., J. rasskasovae as figured by Repina et al., Reference Repina, Lazarenko, Meshkova, Korshunov, Nikiforov and Aksarina1974, pl. 27, fig. 9). Other putative differences based on Palmer and Repina's (Reference Palmer and Repina1993) diagnosis of Judomia (e.g., the absence of intergenal spines) are not considered herein because some Judomia species do bear tiny intergenal spines very similar to those observed in Callavia choffati (e.g., J. rasskasovae as figured by Repina et al., Reference Repina, Lazarenko, Meshkova, Korshunov, Nikiforov and Aksarina1974, pl. 27, fig. 4.5), although in a more proximal position (adaxial to the adgenal angle). Even though we do not agree with Sdzuy's (Reference Sdzuy2001) assignment of ‘Callavia? lotzei’ to the genus Judomia, we do recognize several characters in common, not only in cephalic morphology but also in the thoracic structure (compare Fig. 4.1 with Palmer and Repina, Reference Palmer and Repina1993, fig. 10.7). A close relationship between these taxa was already suggested by Lieberman (Reference Lieberman2001) through a classification that is adopted herein (see remarks on the superfamily).
With regard to other documented occurrences of Callavia, and excluding those listed in Table 1, the genus was identified in the Purley Shales, Warwickshire, by Rushton (Reference Rushton1966) and Williams et al. (Reference Williams, Ruston, Cook, Zalasiewicz, Martin, Condon and Winrow2013), who described as Callavia? sp. a few fragments, mainly based on sculpture similar to that presented by Callavia callavei. Part of this material had been previously documented by Pringle (Reference Pringle1913), Illing (Reference Illing1913, Reference Illing1916), and Smith and White (Reference Smith and White1963). Based on the figured material (Rushton, Reference Rushton1966, pl. 4, figs. 27, 28; Williams et al., Reference Williams, Ruston, Cook, Zalasiewicz, Martin, Condon and Winrow2013, fig. 4), it is not possible to identify them to generic or suprageneric levels, and additional material from those beds is necessary to confirm the presence of Callavia in those levels.
After several misconceptions that indicated the presence of Callavia in the lower Cambrian of Morocco (Neltner and Poctey, Reference Neltner and Poctey1950; Hupé, Reference Hupé1959) these were later reassigned by Geyer and Palmer (Reference Geyer and Palmer1995) to other genera. Geyer and Landing (Reference Geyer and Landing2002) reported the presence of this genus in this region, namely a single cephalic fragment in the Bani section, Moroccan Anti-Atlas (Geyer, personal communication, 2020).
Callavia choffati (Delgado, Reference Delgado1904)
Figures 2–4
- v *Reference Delgado1904
Paradoxides choffati Delgado, p. 319, pl. 1, figs. 1–3, 16?, pl. 5, fig. 3.
- v Reference Delgado1904
Paradoxides sp. aff. P. abenacus Matthew, Reference Matthew1886 var. (?); Delgado, p. 322, pl. 1, fig. 10, pl. 5, fig. 35.
- v Reference Delgado1904
Paradoxides sp. n. aff. P. spinosus Boeck, Reference Boeck1827; Delgado, p. 323, pl. 1, fig. 12.
- v Reference Delgado1904
Paradoxides costae Delgado, p. 323, pl. 1, fig. 6.
- v Reference Delgado1904
Olenellus? macphersoni Delgado, p. 347, pl. 4, fig. 5, pl. 5, fig. 21, pl. 6, fig. 11.
- v Reference Delgado1904
Olenopsis sp.; Delgado, p. 325, pl. 1, fig. 8.
- Reference Richter and Richter1941
Callavia? lotzei Richter and Richter, p. 34, pl. 3, figs. 36–40, pl. 4, fig. 66.
- Reference Richter and Richter1941
Callavia (?) choffati; Richter and Richter, p. 62.
- v Reference Teixeira1952
Callavia choffati; Teixeira, p. 170, pl. 1, fig. 1, pl. 2, figs. 1–3, pl. 3, figs. 1–7, pl. 4, figs. 1–6, pl. 12, figs. 1, 2.
- Reference Sdzuy1962
Callavia? lotzei; Sdzuy, p. 193, pl. 19, figs. 6–14, pl, 22, fig. 11.
- v Reference Liñán and Mergl1982
Callavia? lotzei; Liñán and Mergl, p. 212.
- v Reference Liñán and Mergl1982
Callavia? sp.; Liñán and Mergl, p. 212.
- Reference Sdzuy2001
Judomia lotzei; Sdzuy, p. 96, figs. 25–27.
- v Reference Sdzuy2001
Callavia choffati; Sdzuy, figs. 13, 14.
- Reference Lieberman2001
Sdzuyomia lotzei; Lieberman, p. 113.
- v Reference Collantes, Gozalo, Mayoral, Garzón, Chirivella and Liñán2019
Sdzuyomia lotzei; Collantes et al., p. 81, fig. 2.
Type specimens
Lectotype (selected herein), MG 15781, an internal and external mold of a cephalon (Fig. 2.1), figured by Delgado (Reference Delgado1904, pl. 1, fig. 3) and Teixeira (Reference Teixeira1952, pl. 1). Paralectotypes of one incomplete exoskeleton, MG 15787a with counterpart 15787b (Fig. 4.1; Delgado, Reference Delgado1904, pl. 1, fig. 16, pl. 5, fig. 3; Teixeira, Reference Teixeira1952, pl. 4, figs. 1–3); MG 15782a with counterpart 15782b, five cephala (Fig. 2.2; Delgado, Reference Delgado1904, pl. 1., fig. 1; Teixeira, Reference Teixeira1952, pl. 2, figs. 1–3; MG 15785, external mold (Teixeira, Reference Teixeira1952, pl. 4, figs. 5, 6); MG 15786, internal mold (Fig. 2.5; Delgado, Reference Delgado1904, pl. 1, fig. 1; Teixeira, Reference Teixeira1952, pl. 12, figs. 1, 2); MG 16684, external mold; MG sn, external mold (Fig. 2.3; Teixeira, Reference Teixeira1952, pl. 3, figs. 3, 4); MG 15779a with counterpart 15779b, two cephalic fragments (Delgado, Reference Delgado1904, pl. 4, fig. 53; Teixeira, Reference Teixeira1952, pl. 3, figs. 5, 6; MG 15780a with counterpart 15780b (Teixeira, Reference Teixeira1952, pl. 3, fig. 7); MG 16658, three thoracic segments (all internal molds) (Fig. 4.9; Delgado, Reference Delgado1904, pl. 1, fig. 12); MG 15714 (Fig. 4.8; Delgado, Reference Delgado1904, pl. 1, fig. 8); MG 15764 (Fig. 4.7; Delgado, Reference Delgado1904, pl. 1, fig. 6); MG 15707a with counterpart 15707b, one pygidium (Fig. 4.6; Delgado, Reference Delgado1904, pl. 1, fig. 10, pl. 5, fig. 35).
Emended diagnosis
Intergenal spine reduced; S4 poorly defined and short (tr.); interocular area width approximately four-fifths width of the extraocular area opposite S1; intergenal ridge and posterior ocular line equally prominent, subparallel to the intergenal spine. Thorax of 18 or probably 19 segments, tapering posterior to fifth segment.
Occurrence
Vila Boim Formation (type locality), Marianian (Cambrian Series 2) of Vila Boim, Portugal; lower part of Cumbres beds, middle Marianian (Cambrian Series 2), Cumbres de San Bartolomé, Huelva, Spain; and lower part of Herrerías shale, middle Marianian (Cambrian Series 2), Sierra del Bujo (Arroyomolinos de León), Cañaveral de León and Hinojales, Huelva, Spain.
Description
Cephalon crescent-shaped; sagittal length ~40% of maximum width at posterior margin, with higher relief of the anterior border, the ocular ridges, and the glabella. Known cephala range 2.1–18.9 mm length and 5.2–42.4 mm width. Posterior margin of cephalon curved backward distally. Glabella hourglass-shaped to parallel-sided, with faint constriction at S1, inflated dorsally, exceeding the genal areas in lateral view; maximum glabellar elevation at half of glabellar length, sloping downward anteriorly with rounded profile. Glabella longer than wide; posterior glabellar width ~115–120% the anterior glabellar width; corresponding to 25% of maximum cephalic width; sagittal glabellar length ~75–80% sagittal cephalic length. Axial furrows deep, slightly sinuous (outlining glabellar lobes). Occipital furrow moderately deep to shallow medially, oblique backward when traced adaxially; occipital ring moderately convex dorsally, frequently preserved as two symmetrical subrectangular lobes, bearing a small axial node near posterior margin. Four glabellar furrows (excluding occipital furrow) shallowing anteriorly, nontransglabellar, nearly straight, oblique, inward and backward ~10–15° to a transverse line. S1 subparallel to SO, occupying approximately two-thirds of glabellar width; L1 subrectangular, moderately inflated dorsally; S2 slightly less oblique than S1; L2 similar to L1; S3 subparallel to S2 but shorter (tr.); L3 shorter (exsag.) and narrower (tr.) than L1 and L2; S4 poorly defined, very shallow and narrow (tr.), located immediately posterior to the parafrontal band; L4 poorly defined, typically merged with the frontal lobe (Fig. 3.17). Frontal lobe of glabella tapered to slightly pointed, surrounded by parafrontal band connecting ocular ridges. Preglabellar field short, < 10% of sagittal cephalic length. Some specimens showing shallow furrow connecting preglabellar furrow with anterior border furrow (Fig. 3.7). Lateral border inflated dorsally, widened posteriorly, reaching maximum width at base of genal spines, defining broad genal point, directed backward. Anterior and lateral border furrows broad, deep, merging into significantly shallower posterior border furrow, but deeper at genal areas. Interocular area dorsally arched, elongated (exsag.), trapezoidal in outline, with two differentiated interocular swellings. Width (tr.) of interocular area approximately four-fifths of extraocular area width at S1 level. Ocular lobe prominent, arc-shaped, located slightly closer to glabella than to lateral border; exsagittal length equivalent to 40% of sagittal cephalic length. Posterior tip of ocular lobe opposite SO; anterior tip opposite L3. Inner margin of ocular lobe well defined. Ocular lobes anteriorly connected by parafrontal band, posteriorly connected to posterior ocular line. Pronounced intergenal ridge, slightly curved to sigmoidal, extending into reduced, almost indistinct, intergenal spine (Fig. 3.8, 3.18. 3.20), giving rise to change of convexity of posterior margin (small dorsal swelling). Intergenal spine located in exsagittal line with inner edge of lateral border furrow. Genal angle greater than intergenal angle. Sculpture composed of reticulated pattern, with extraocular genal caeca, and terrace ridges along abaxial limit of anterior border (Figs. 2.4, 3.19).
Thorax composed of 18, probably 19 thoracic segments in only complete specimen (Fig. 4.1, 4.2). Wide (sag.), convex, little-pronounced axial rings, narrowing toward back. Axial ring width (tr.) occupying 20% of total thoracic width anteriorly, 35% posteriorly. Axial ring furrows deep. Axial nodes on each thoracic segment. Lateral extension of pleural region not surpassing extension of genal spine of cephalon. Pleural region subtly widened (tr.) to third pleura, then progressively narrowing (tr.) toward posterior end. Pleurae thin (sag.), knife-shaped, slightly curving backward; curvature progressively increasing posteriorly. Pleural furrow deep, wide, extending 35% of whole pleura (tr.), slightly oblique. Curvature located at approximate midlength of pleural width, with pleural spine widened (exsag.) at that point. Last segments of thorax fused with pygidium.
Pygidium very reduced, relatively narrow. Pygidial rachis well-defined, with triangular outline, composed of three faint axial rings. Pleural regions poorly preserved, posteriorly extended.
Materials
Type specimens plus additional material: Cumbres beds, Cumbres de San Bartolomé: UHU-CU1/1/1, 1/2/3, two cephala.
Herrerías shale: Hinojales: UHU-LH1/1/8, 1/1/12, 1/1/26, 1/2/2, 1/2/7, 1/2/24, 1/2/25, seven cephala; UHU-LH1/1/4, thoracic segment. Cañaveral de León: UHU-CVL36 (Fig. 4.3), 37 (Fig. 4.5), 49 (Fig. 4.2), three articulated incomplete cephalothoraxes; UHU-CVL01−13, 15−35, 38−43, 46−48, 50, 44 cephala. Sierra del Bujo: UHU-LBU/0/1, /0/2, /0/3, /1/1, four cephala; UHU-LBU/0/2, cephalic fragment.
Remarks
Callavia choffati differs from the type species Callavia broeggeri and Callavia callavei in having shorter and narrower intergenal spines, shorter (tr.) interocular areas than in Callavia broeggeri, and a different glabellar lobation (fainter glabellar furrows and shorter S3 and S4) than in Callavia callavei.
A group of specimens described by Delgado (Reference Delgado1904) under different species names are conspecific with ‘Paradoxides’ choffati, as previously stated (e.g., Richter and Richter, Reference Richter and Richter1941; Teixeira, Reference Teixeira1952; see synonymy for further information). Callavia choffati was originally described by Delgado (Reference Delgado1904) as a species of Paradoxides and quickly after his publication, Charles Schuchert (in Dana, Reference Dana1905, p. 159) stated that “Paradoxides choffati is clearly an Olenellus.” In fact, Delgado (Reference Delgado1904, p. 320) expressed the same opinion in the original publication: “… ces exemplaires pourraient être exclus du genre Paradoxides (s. str.) et réunis plutôt à Olenellus, sous-genre Holmia …” It was the observation of ‘clear’ facial sutures in Delgado's (Reference Delgado1904) opinion, especially in the specimen herein selected as lectotype (Fig. 2.1), which justified the assignment to Paradoxides. Today, it is widely agreed that these cephalic ‘lines’ observed in many specimens of Olenellina are in fact fractures (Whittington, Reference Whittington1989, Reference Whittington1997), and that the suborder Olenellina is characterized by lacking dorsal sutures throughout its entire ontogeny. But during several decades, the regular presence in olenellines and the curious configuration of these lines (which mimic the anterior branches of a facial suture) led to much discussion about their significance (e.g., Størmer, Reference Størmer1942; Hupé, Reference Hupé1953; Bergström, Reference Bergström1973). As Geyer (Reference Geyer1996) stated, they could represent favored loci for fracturing due to thinner or less calcified cuticle. In the studied material of Callavia choffati, these fractures are, in fact very common (Fig. 3.7, 3.10, 3.11, 3.13–3.19).
Some decades after Delgado's (Reference Delgado1904) publication, Richter and Richter (Reference Richter and Richter1941) erected a new species, Callavia? lotzei, from the northern Huelva province (Spain). These authors suggested for the first time that ‘Paradoxides’ choffati should be also assigned to the genus Callavia. Nevertheless, and although they considered it very similar to the newly erected Callavia? lotzei, they did not specify the morphological features that justified the erection of a new species. Furthermore, Richter and Richter (Reference Richter and Richter1941) considered that Portuguese and Spanish Callavia-bearing assemblages are coeval and share some taxa at specific levels, thus correlation would benefit from such discussion and eventual synonymy. While revising Delgado's (Reference Delgado1904) material, Teixeira (Reference Teixeira1952) followed Richter and Richter's (Reference Richter and Richter1941) suggestion and maintained the Portuguese species assigned to the genus Callavia. A few years later, Hupé (Reference Hupé1960) suggested that Callavia? lotzei and Callavia choffati might be synonyms, but Sdzuy (Reference Sdzuy1962) rejected this hypothesis based on deeper glabellar furrows and larger cephala of Callavia choffati. Furthermore, Sdzuy (Reference Sdzuy1962) considered that both Iberian species bear significant differences when comparing with Callavia, namely the lack of intergenal spines and the absence of a large occipital spine (both structures are indeed present but based on the poorly preserved material that Sdzuy had at his disposal, it was not possible to verify them). For these reasons, Sdzuy (Reference Sdzuy1962) suggested that Callavia choffati was more closely related to Kjerulfia Kiær, Reference Kiær1917, whereas Callavia lotzei should be assigned to Judomia, with which it shares several morphological characters, although he maintained both species as Callavia?. This proposed assignment was later reinforced by the author (Sdzuy, Reference Sdzuy2001), who definitely transferred the Spanish species to the genus Judomia, stating that it was unexpected because Judomia is characteristic of the Siberian domain. Among the characters that Sdzuy (Reference Sdzuy2001) listed in common, are the absence of intergenal spines and the pygidium configuration, which he considered to be much more similar to those of Judomia than Callavia. Nevertheless, the intergenal spines are present in Callavia lotzei, and Whittington (Reference Whittington1989, p. 134) stated that the “pygidium attributed to Callavia by Raw (Reference Raw1936, pl. 21, figs. 3a–c) is composed largely of one pair of spines similar in form to those of” Judomia tera. In that work, Sdzuy (Reference Sdzuy2001) also reinforced the independence of Callavia choffati and Callavia lotzei through photographic retrodeformation of Portuguese figured specimens, but the results are not reliable (Sdzuy, Reference Sdzuy2001, figs. 13, 14).
The new data described in this work suggest that Callavia choffati and Callavia lotzei are synonyms and support the assignment of the Iberian species to the genus Callavia. The previous poor documentation of Callavia choffati from the Vila Boim Formation (Portugal), namely short descriptions and low resolution photographs (Delgado, Reference Delgado1904; Teixeira, Reference Teixeira1952), together with the large size of the available specimens, certainly hinder proper comparison with ‘Callavia? lotzei’ from the Herrerías shale (Spain). On the other hand, in erecting ‘Callavia? lotzei,’ Richter and Richter (Reference Richter and Richter1941) included specimens in this species belonging to Gigantopygus cf. G. bondoni Hupé, Reference Hupé1953, a redlichiine, and Sdzuy (Reference Sdzuy1962, pl. 19, figs. 7–11) included some additional material of meraspides. These compromised comparison with the Portuguese material. The great number of newly collected, well-preserved specimens from the Herrerías shale allowed clarification of the morphology of ‘Callavia? lotzei’ and verification that it agrees with Callavia choffati in all characters. Reduced intergenal spines are present in both sets of specimens, located in similar positions (e.g., Fig. 3.1–3.17). This structure seems to be progressively reduced to a tiny spine or node on the posterior cephalic border through ontogeny, being larger and more evident in small specimens (Fig. 3). Due to the deformation and large size of the type specimens (Fig. 2), the intergenal spine is not evident, being expressed as a swelling on the posterior border, and more evident in only one specimen (Fig. 2.3). The inflated cephalic border, widening posteriorly, is one of the characters that Sdzuy (Reference Sdzuy2001) considered to separate Portuguese and Spanish forms; it has the same configuration in all of the studied specimens. The apparent relatively shorter cephalic border in the Portuguese material (Fig. 2.1–2.5) could be attributable to differences in size due to ontogenetic allometry. Another important comparative character is the glabellar morphology and lobation; both sets of specimens show a constriction opposite S1 (Figs. 2.1, 3.17, 3.19), resulting in a faint hourglass-shaped glabella in specimens preserving part of the original convexity. The outline and position of the ocular lobes are indistinguishable in Portuguese and Spanish specimens, including the typical swellings of the interocular areas (e.g., Figs. 2.1, 2.2, 3.11, 3.17). Furthermore, both bear an entirely comparable, faint parafrontal band surrounding the LA (Figs. 2.1–2.3, 3.7, 3.13–3.18) and a well-marked posterior ocular line subparallel to the intergenal ridge (Figs. 2.1, 3.18, 3.19). The few articulated specimens also show the same thoracic structure (compare Sdzuy, Reference Sdzuy1962, pl. 9, fig. 6 with Fig. 4.1). For these reasons, ‘Callavia? lotzei’ is herein considered a junior synonym of Callavia choffati.
Paleobiogeographical and biostratigraphical remarks
The confirmed presence of the genus Callavia in the Iberian Peninsula, represented by the species Callavia choffati, together with its reported occurrence in Morocco (Geyer and Landing, Reference Geyer and Landing2002), are important with regard to the early Cambrian faunal links between the western Gondwana Domain and Avalonia. The genus Callavia (in its current concept), originally described from the Brigus Formation, Newfoundland, Canada (Walcott, Reference Walcott1890; Matthew, Reference Matthew1897), was later identified in the Comley Limestone Formation, Shropshire, UK (Lapworth, Reference Lapworth1891), and soon thereafter became an index taxon for the Avalonian realm up to this day. Based on associated trilobites from the Brigus Formation in eastern Newfoundland and the Comley section of England (Triangulaspis Lermontova, Reference Lermontova1940, Delgadella Walcott, Reference Walcott1912, Serrodiscus bellimarginatus [Shaler and Foerste, Reference Shaler and Foerste1888], it was possible to approximately correlate the so-called Callavia Biozone (Avalonian regional Branchian Series, lower Cambrian, Series 2) with other paleogeographical regions (the Banian/Marianian regional Stages of Morocco/Iberia, the ‘Nevadella’ Biozone of Laurentia, the Pagetiellus anabarus-Judomia Biozones of Siberia, or the Schmidtiellus mickwitzi-Holmia inusitata-Holmia kjerulfi Biozones of Baltica; see Sdzuy, Reference Sdzuy1971, Reference Sdzuy1972; Palmer and Repina, Reference Palmer and Repina1993, fig. 12; Fletcher, Reference Fletcher2006; Żylińska, Reference Żylińska2013; Sundberg et al., Reference Sundberg, Geyer, Kruse, McCollum, Pegel, Zylinska and Zhuravlev2016). The type species Callavia broeggeri is a representative of the ‘west Avalonia sector’ (= American sector), which includes the eastern North American seaboard from Newfoundland as far south as Cape Cod, Massachusetts (Cocks and Torsvik, Reference Cocks and Torsvik2006), and is present throughout this area (e.g., Grabau, Reference Grabau1900; Walcott, Reference Walcott1910; Fletcher, Reference Fletcher2003, Reference Fletcher2006). On the other hand, the ‘eastern sector of Avalonia’ (= European sector) is represented by the presence of Callavia callavei and several findings of Callavia sp. indet., a species occurring in Branchian sequences in England (Thomas et al., Reference Thomas, Owens and Rushton1984; Rushton, Reference Rushton1999; Rushton et al., Reference Rushton, Brück, Molyneux, Williams and Woodcock2011; Williams et al., Reference Williams, Ruston, Cook, Zalasiewicz, Martin, Condon and Winrow2013).
During the early Cambrian, and probably until the end of this period (Cocks and Torsvik, Reference Cocks and Torsvik2006; Pouclet et al., Reference Pouclet, Aarab, Fekkuk and Benharref2007), Avalonia was aggregated to the margin of West Gondwana, possibly belonging to the same biochorema as Iberia, which was a peri-Gondwanan terrane located east of Avalonia at the same western margin (Courjault-Radé et al., Reference Courjault-Radé, Debrenne and Gandin1992; fig. 5). According to Álvaro et al. (Reference Álvaro, Ahlberg, Babcock, Bordonaro, Choi, Cooper, Ergaliev, Gapp, Ghobadi Pour, Hughes, Jago, Korovnikov, Laurie, Lieberman, Paterson, Pegel, Popov, Rushton, Sukhov, Tortello, Zhou and Żylińska2013, p. 285), the end of Cambrian Series 2 is characterized by “new links between Avalonia and West Gondwana, including some eodiscoids and species of Protolenus, Strenuella and possibly Callavia.” Our data confirm the presence of Callavia in Iberia, which, together with the Moroccan occurrence of the genus (Geyer and Landing, Reference Geyer and Landing2002), supports the faunal links between both regions and is in agreement with the ideas of previous authors, who reported several other genera in common from Cambrian Series 2 onward between Avalonia and the western Mediterranean region (e.g., Sdzuy, Reference Sdzuy1972; Liñán et al., Reference Liñán, Gozalo, Palacios, Gámez Vinaned, Ugidos, Mayoral, Gibbons and Moreno2002; Álvaro et al., Reference Álvaro, Elicki, Geyer, Rushton and Shergold2003; Landing et al., Reference Landing, Geyer, Brasier and Bowring2013a, Reference Landing, Westrop and Bowringb, Collantes et al., Reference Collantes, Mayoral, Liñán and Gozalo2021). Therefore, the genus Callavia is distributed across the western margin of Gondwana, the western Mediterranean region (Iberia and Morocco), and throughout all of the Avalonian sectors (UK, Newfoundland, and Massachusetts).
With regard to the age of the studied assemblages and their correlation with other regions, the lower part of the Vila Boim Formation (Portugal) has been assigned to the middle part of the regional Marianian Stage (see Liñán et al., Reference Liñán, Perejón, Gozalo, Moreno-Eiris and Oliveira2004). In Spain, the newly collected material and the previous documented specimens of ‘Callavia? lotzei’ (= Callavia choffati) by Richter and Richter (Reference Richter and Richter1941) and Sdzuy (Reference Sdzuy1962, Reference Sdzuy2001), all come from the northern Huelva province, in rocks assigned to the Cumbres beds and the Herrerías shale, with an age corresponding to the middle Marianian (Ruiz López et al., Reference Ruiz López, Fernández Carrasco, Collaut Saenz de Sicilia and Apalategui1979), coeval with Portuguese levels. The middle part of the Marianian regional Stage can be correlated with the uppermost Cambrian Stage 3 to the lowermost Cambrian Stage 4 (Zhang et al., Reference Zhang, Ahlberg, Babcock, Choi, Geyer, Gozalo, Hollingsworth, Li, Naimark, Pegel, Steiner, Wotte and Zhang2017; Collantes et al., Reference Collantes, Mayoral, Chirivella and Gozalo2020, Reference Collantes, Mayoral, Liñán and Gozalo2021). In the Avalonian sectors, Callavia broeggeri is known from the Brigus Formation and coeval levels (Purley Shales), assigned to the lower Branchian Series (top of Cambrian Stage 3 to the base of Cambrian Stage 4; Landing, Reference Landing1996; Fletcher, Reference Fletcher2003, Reference Fletcher2006), whereas Callavia callavei and several findings of Callavia sp. indet. occur in the Comley Limestone Formation (England; Thomas et al., Reference Thomas, Owens and Rushton1984; Rushton, Reference Rushton1999; Williams et al., Reference Williams, Ruston, Cook, Zalasiewicz, Martin, Condon and Winrow2013). All of these levels correspond to the Callavia Zone and Strenuella sabulosa Biozone, equivalent to uppermost Cambrian Stage 3 to lowermost Cambrian Stage 4 (Rushton et al., Reference Rushton, Brück, Molyneux, Williams and Woodcock2011; Williams et al., Reference Williams, Ruston, Cook, Zalasiewicz, Martin, Condon and Winrow2013).
Specimens of Callavia? sp. indet. collected by Illing (Reference Illing1913), as well as those described and figured by Rushton (Reference Rushton1966), were obtained from calcareous nodules at the base of the Purley Shales at Camp Hill Grange Quarry (northwestern Nuneaton), at Woodlands Quarry (Hartshill) and from Worthington Farm, UK. Later, Brasier (Reference Brasier1984) also obtained fragments of Callavia? sp. indet. in Nuneaton.
All of these Callavia-bearing localities and beds are equivalent to uppermost Cambrian Stage 3 to lowermost Cambrian Stage 4, reinforcing partial correlation between the Marianian/Banian regional Stages and the lower Branchian Series (e.g., Geyer, Reference Geyer2019) and suggesting that the Avalonian Callavia Zone can also have some usefulness in West Gondwana.
Conclusions
A systematic reassessment of ‘Paradoxides’ choffati from Portugal and ‘Callavia? lotzei’ from Spain has placed ‘Callavia? lotzei’ as a junior synonym of ‘P. choffati’ and led to the assignment of the Iberian taxon to Callavia. In turn, Sdzuyomia is considered to represent a junior synonym of Callavia, and the systematic position of this classic genus among Olenellina is better framed within the superfamily ‘Judomioidea.’
Based on the revised diagnosis of Callavia, the genus is distributed across the western margin of Gondwana, the western Mediterranean region (Iberia and Morocco; ‘West Gondwana’), and throughout all of the Avalonian sectors (UK, eastern Newfoundland, and Massachusetts), supporting faunal links between West Gondwana and Avalonia during Cambrian Series 2. The Iberian records of Callavia choffati are assigned to the middle part of the regional Marianian Stage (uppermost Cambrian Stage 3 to lowermost Cambrian Stage 4) and correlate with the Callavia Zone of Avalonia (lower Branchian Series), suggesting some usefulness of this biostratigraphical zone also in West Gondwana and strengthening correlation of the Marianian and Banian regional Stages with the lower Branchian Series.
Acknowledgments
We are grateful to M. Webster (Chicago), F.A. Sundberg (Arizona), and J.O.R. Ebbestad (Uppsala, Sweden) for their detailed reviews and constructive suggestions; and N. Hughes (California) for his efforts in editing the article. We thank the Council of Environment and Territorial Planning from the Junta de Andalucía and the Directorate of Sierra de Aracena y Picos de Aroche Natural Park for providing permission to work into the park; M. Gordo for her contribution with the facilities during the fieldwork campaigns in northern Huelva province; I. Garzón for his fieldwork assistance in Cañaveral de León; M. Ramalho (Museu Geológico de Lisboa), in loving memory, for granting access to fossil collections under his care; J. Colmenar (Centro de Geociências de Coimbra) and the remaining ‘Delgaditos Team’ for assisting in fieldwork in Vila Boim. The present work has been carried out with Junta de Andalucía to the RNM 276 Research Group and the Centro Científico-Tecnológico de Huelva to the Department of Applied Geosciences, together with the GIUV2017-395 research group of the University of Valencia. This study was supported by Portuguese funds by Fundação para a Ciência e a Tecnologia, I.P. (Portugal) in the frame of UID/Multi00073/2019, UIDB/00073/2020 and UIDP/00073/2020 projects of the I & D unit Geosciences Center (CGEO). This work is a contribution to the project IGCP 652.









