Article contents
The collision cross-sections for proton–argon interaction based on ab initio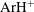 $\text{ArH}^{+}$ potential
$\text{ArH}^{+}$ potential
Published online by Cambridge University Press: 12 March 2020
Abstract
The classical collision cross-sections of a proton with an argon atom as well as the thermal transport coefficients and rate constant of the colliding  $\text{H}^{+}-\text{Ar}$ system are evaluated at the kinetic temperature
$\text{H}^{+}-\text{Ar}$ system are evaluated at the kinetic temperature 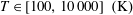 $T\in [100,10\,000]~(\text{K})$ by means of the asymptotically correct analytical potential constructed for the ground
$T\in [100,10\,000]~(\text{K})$ by means of the asymptotically correct analytical potential constructed for the ground 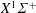 $X^{1}\unicode[STIX]{x1D6F4}^{+}$ state of the ArH+ cation from the highly accurate ab initio data available in the entire range of internuclear distances (Terashkevich et al., J. Quant. Spectrosc. Radiat. Transfer, vol. 234, 2019, pp. 139–146). The results can be useful to estimate thermodynamic, transport and kinetic properties of the Ar/H2 plasma in a wide temperature range.
$X^{1}\unicode[STIX]{x1D6F4}^{+}$ state of the ArH+ cation from the highly accurate ab initio data available in the entire range of internuclear distances (Terashkevich et al., J. Quant. Spectrosc. Radiat. Transfer, vol. 234, 2019, pp. 139–146). The results can be useful to estimate thermodynamic, transport and kinetic properties of the Ar/H2 plasma in a wide temperature range.
Keywords
Information
- Type
- Research Article
- Information
- Copyright
- © Cambridge University Press 2020
References
- 1
- Cited by

