Introduction
Oropharyngeal squamous cell carcinoma (SCC) is a common head and neck cancer, traditionally linked to tobacco and alcohol, but increasingly associated with HPV, especially in younger patients. Reference Barsouk and Aluru1 p16-negative SCCs carry a poorer prognosis and pose therapeutic challenges. Reference Jamal and Anjum2
For locally advanced cases, the standard treatment is radiotherapy (RT) with cisplatin-based chemotherapy (66–70Gy in 30–35fractions). 3 Patients unfit for chemotherapy require RT-only strategies.
Hyperfractionated RT improves outcomes in patients unsuitable for concurrent therapy, Reference Lacas and Bourhis4 yet the need for multiple daily fractions strains scheduling, staff, and patients. Intensity-modulated RT (IMRT) sharpens dose conformity and spares organs at risk (OARs) but leaves those logistical pressures unchanged. Combining IMRT with a simultaneous integrated boost (SIB) creates a practical compromise: high-risk sub-volumes receive an escalated dose within each standard fraction, eliminating a separate sequential boost and shortening the overall course. This approach retains the radiobiologic advantages of acceleration without extra appointments, but it requires meticulous planning and stringent quality assurance to prevent undue toxicity. Reference Orlandi and Palazzi5
Contemporary research is exploring biology-guided “dose-painting,” selectively escalating the dose to radio-resistant, hypoxic sub-volumes delineated on PET/CT or functional magnetic resonance imaging (MRI); while promising, this approach requires sophisticated imaging, contouring, and planning resources, limiting its widespread adoption. Reference Dolezel and Slavik6
This report presents a practical approach: selective dose escalation to the central gross tumor volume (GTV) using volumetric modulated arc therapy (VMAT) and SIB, without functional imaging. By targeting the hypoxia-prone tumor core, this method potentially maintains efficacy while reducing toxicity compared to whole-tumor boosting.
Case Description
In January 2020, a 66-year-old man with p16-negative SCC of the soft palate and bulky cervical lymphadenopathy (cT1N3bM0) was assessed by the multidisciplinary tumor board at Fondazione Policlinico Universitario Agostino Gemelli. He had a history of alcohol-related liver cirrhosis with ascitic decompensation, making him unfit for concurrent chemotherapy. He was a former smoker and consumed alcohol moderately. Clinical examination revealed an approximately 1 cm erythroplakia-like lesion on the soft palate. Biopsy confirmed well-to-moderately differentiated SCC (G1/G2) with basaloid features, positive for p63, p40, and CK14, negative for p16, and a Ki-67 index of ∼35%. Fine-needle aspiration of a right cervical lymph node confirmed metastatic SCC. Contrast-enhanced CT of the head, neck, and thorax revealed a large, cystic necrotic lymph node metastasis in the right neck, measuring 4.8 × 3.8 cm with extracapsular extension (Figure 1), but no evidence of distant metastases. The primary tumor was not clearly delineated on imaging, likely due to post-biopsy mucosal enhancement. MRI was contraindicated due to a metallic hip prosthesis. Given his preserved performance status (ECOG0) and the tumor profile, the decision was made to proceed with definitive RT alone, in line with recommended treatment strategies for p16-negative oropharyngeal SCC in patients who cannot undergo chemoradiotherapy. 3
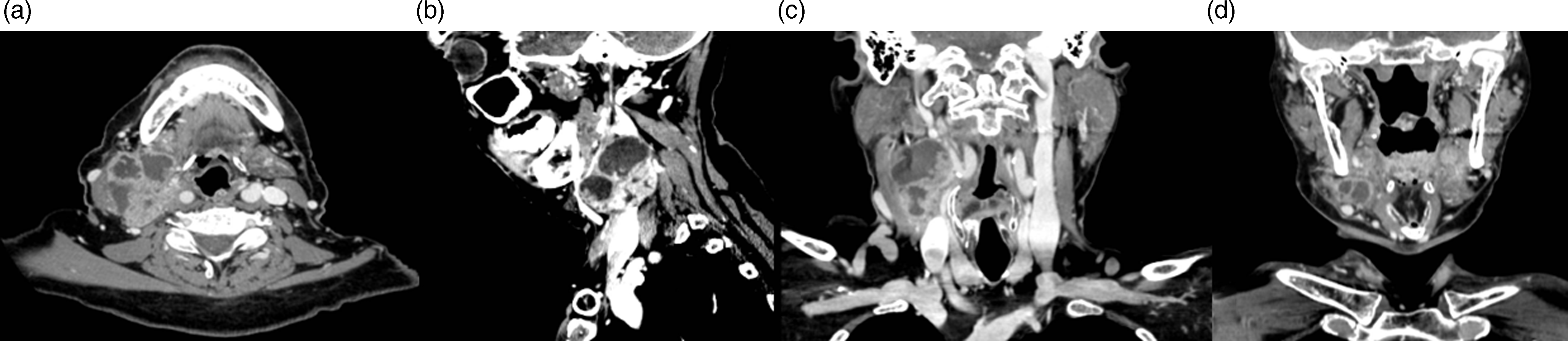
Figure 1. Contrast-enhanced CT images showing the large cystic-necrotic right laterocervical lymph node metastasis in a patient with p16-negative oropharyngeal squamous cell carcinoma. (a) Axial view shows metastasis involving neck levels II and III with extracapsular spread. (b) Sagittal view highlights the extent of the nodal disease and its relationship with surrounding structures. (c, d) Coronal views depict the size and infiltration of the nodal metastasis, showing its proximity to the mandible and cervical vasculature.
Treatment Plan
Following dental clearance and CT simulation with thermoplastic mask immobilization, the patient was treated with volumetric-modulated arc therapy (VMAT) using four 6MV arcs on a Varian EDGE linear accelerator. An adaptive simultaneous integrated boost (SIB) technique was employed, accounting for tumor burden and risk of microscopic spread:
-
Boost volume (GTVboost): the innermost 5-millimetre, presumed hypoxic core of the bulky nodal GTV is escalated to 72Gy delivered in 30 fractions (2.4Gy per fraction), a one–week–accelerated, moderately hypofractionated regimen.
-
High-risk volume (CTV_66): 66Gy in 30 fractions (2.2Gy/fraction) covering gross disease and primary tumor bed (soft palate, right tonsil).
-
Intermediate-risk volume (CTV_60): 60Gy in 30 fractions (2.0Gy/fraction) to intermediate-risk nodal levels (right Ib, II, III, VIIa-b).
-
Low-risk volume (CTV_54): 54Gy in 30 fractions (1.8Gy/fraction) to low-risk nodal levels (left Ib–III, right IV–V).
A 3 mm margin was added to each CTV (excluding the inner GTV) to form PTVs. Dose coverage met planning goals, with 95% of each volume receiving ≥ 95% of the prescribed dose. OARs—including spinal cord, carotids, mandible, oral cavity, and pharyngeal constrictors—were contoured and prioritized according to current SIB-IMRT guidelines. Reference Merlotti and Alterio7 Dose distributions are presented in Figures 2 and 3.
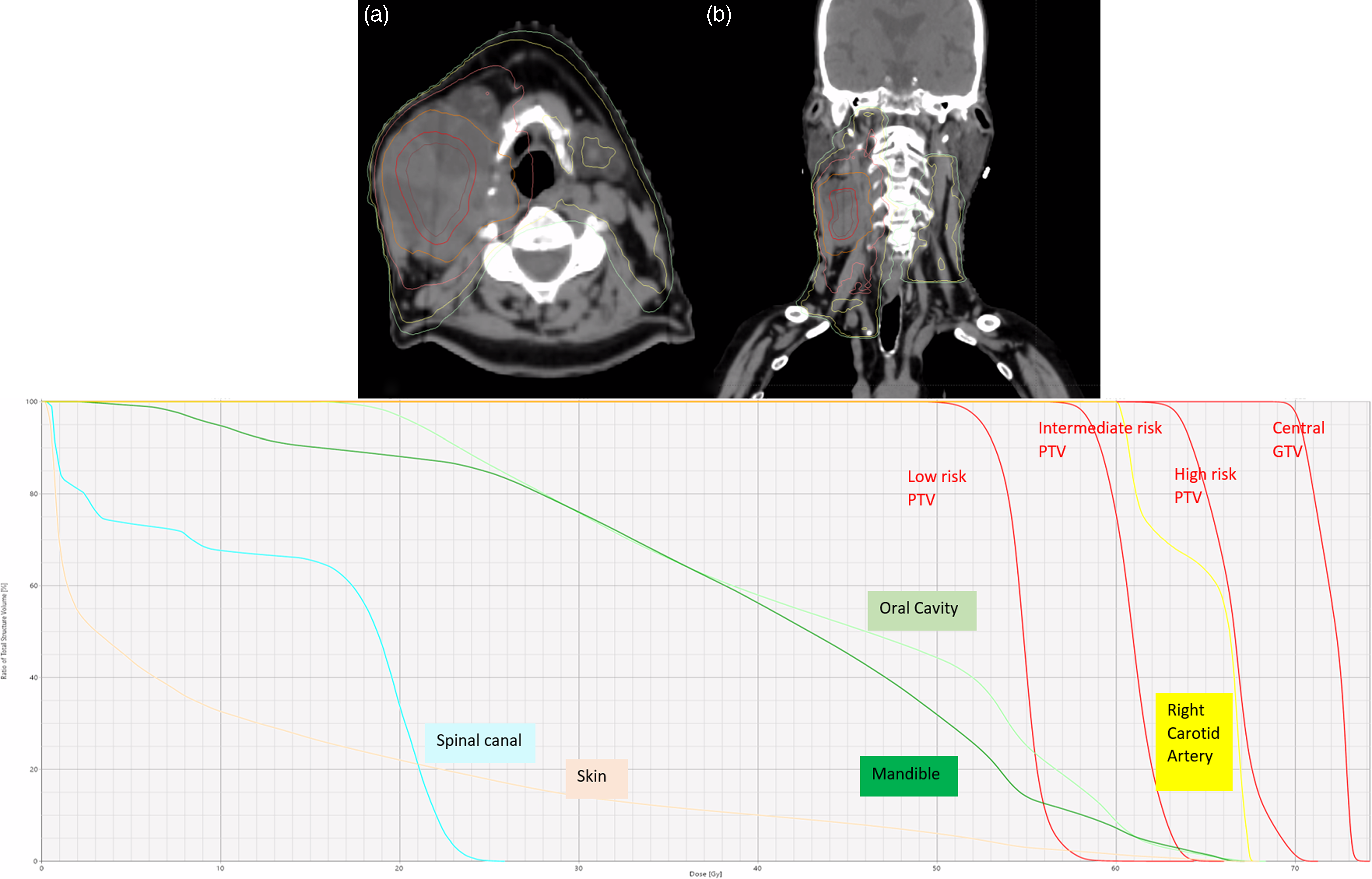
Figure 2. Dose distribution and dose-volume histogram (DVH) for the VMAT plan using Simultaneous Integrated Boost (SIB) shown in axial (a) and coronal (b) CT views. Isodose lines represent dose escalation levels: 72 Gy (red), 66 Gy (orange), 60 Gy (pink), and 54 Gy (yellow/green), targeting the central GTV. The DVH demonstrates that organs at risk (OARs) such as the spinal cord, carotid artery, oral cavity, and mandible receive significantly lower doses compared to the central GTV. This highlights the precision of tumor targeting while effectively minimizing radiation exposure to surrounding healthy tissues.
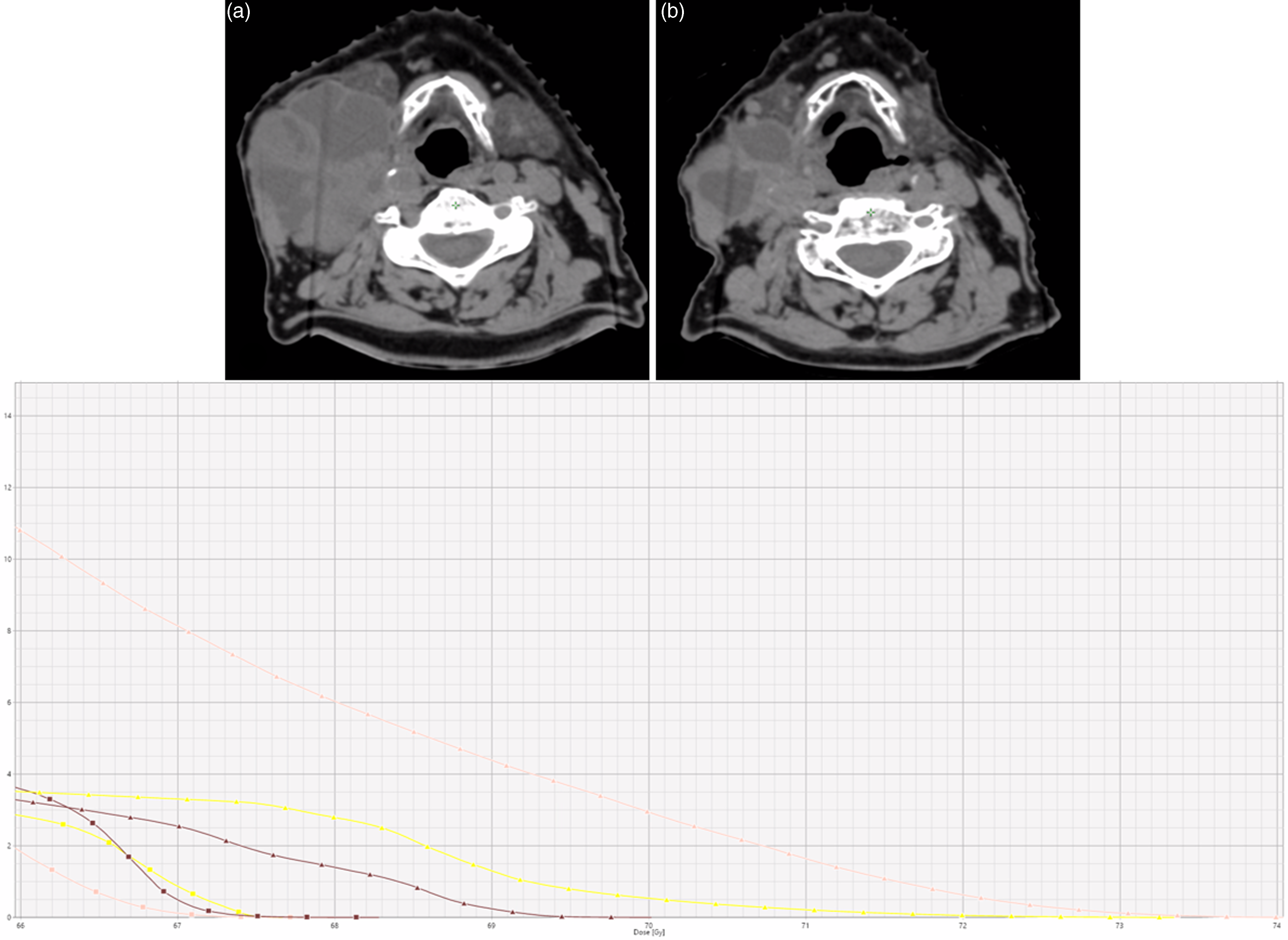
Figure 3. Axial CT images showing dose distribution and tumor response during treatment. (a) Pretreatment image shows a large, bulky right laterocervical lymph node with cystic-necrotic components. (b) Mid-treatment image shows significant tumor shrinkage following radiotherapy. The lower panel displays the dose-volume histogram (DVH), illustrating the volume of organs at risk (OARs) receiving doses above 66 Gy. Skin (pink), constrictor muscles (brown), and right carotid artery (yellow) are highlighted, comparing the initial planning (squares) with the dose distribution during treatment without adaptive replanning (triangles), showing increased OAR exposure due to tumor shrinkage.
Treatment Delivery
Daily image guidance with cone beam CT (CBCT) ensured accurate alignment throughout treatment. As part of the institutional protocol for radical head and neck radiotherapy, a mid-treatment planning CT was performed, revealing substantial tumor shrinkage. The nodal GTV decreased from 107 cc to 33 cc, prompting adaptive replanning. The revised plan improved dose conformity to the regressed tumor and enhanced sparing of nearby structures, as illustrated in Figure 4. After the 27th fraction, the patient developed grade 3 moist desquamation (per CTCAE v5.0), resulting in a four-day interruption. Supportive care measures—including topical therapy and wound management—were provided. The patient subsequently resumed treatment and completed radiotherapy within 48 days without further delays.
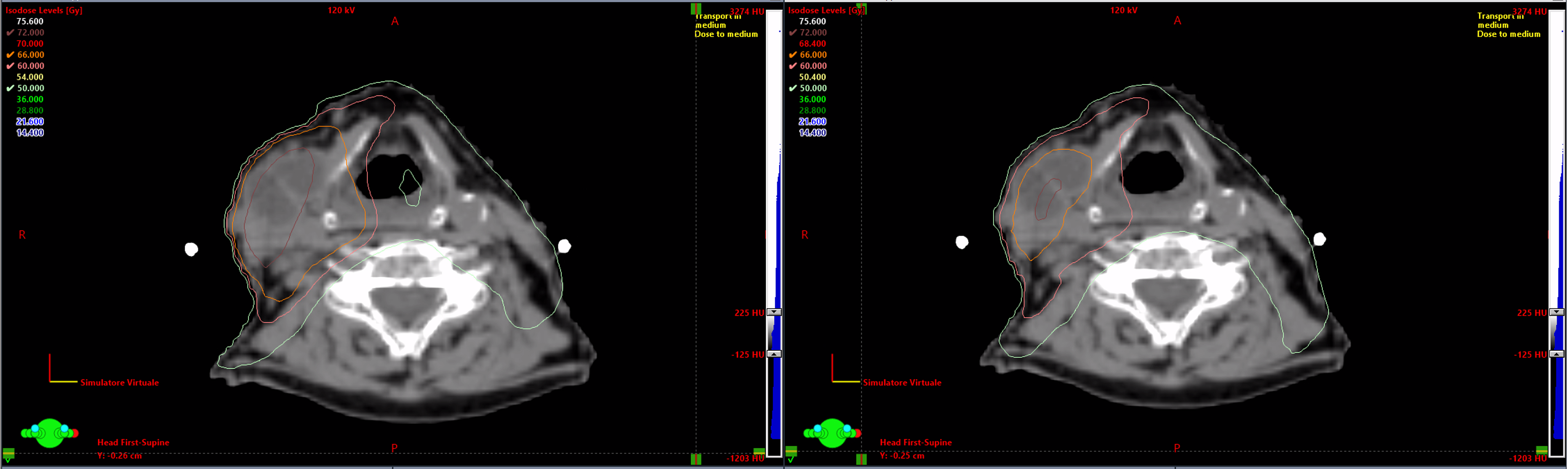
Figure 4. Axial CT images showing isodose distributions before (left) and after (right) adaptive re-planning. Tumor shrinkage allowed for dose redistribution, improving conformity and sparing organs at risk.
Clinical Outcome
Three months posttreatment, contrast-enhanced CT showed significant tumor regression. PET-CT confirmed a complete metabolic response within the first year. At five-year follow-up, the patient remains in complete remission with no recurrence. He maintains ECOG 0 status, good nutritional condition, and reports only mild dysphagia for solids since year three. No late complications such as osteoradionecrosis or vascular injury were observed.
Discussion
This case highlights the potential of VMAT with SIB in the treatment of bulky nodal disease in a patient with p16-negative oropharyngeal SCC who was ineligible for chemotherapy. The strategy allowed for effective tumor control through dose escalation while maintaining a focus on organ-at-risk (OAR) sparing, consistent with modern principles of precision radiotherapy.
While tumor subvolume boosting guided by functional imaging (e.g., FMISO-PET or dynamic contrast-enhanced MRI) is a growing area of research, clinical implementation remains limited by factors such as cost, availability, and the changing nature of tumor hypoxia over time. Reference Dolezel and Slavik6 In this context, central boosting to the core of the nodal GTV offers a pragmatic alternative. The tumor core is often considered more hypoxic and radioresistant; thus, targeting it may approximate the benefits of biologically guided dose painting without requiring advanced imaging tools.
Compared to whole-tumor SIB approaches, the selective central GTV boost reduces the extent of high-dose exposure to adjacent mucosa and other critical structures. However, the use of hypofractionation in this setting demands careful attention. As observed by Verduijn et al., hypofractionated boosts have been associated with increased rates of post-radiation mucosal ulceration (PRMU), particularly in mucosal tumors. Reference Verduijn and Petit8 In our patient, the development of grade 3 moist desquamation required a brief treatment interruption, reinforcing the importance of adhering to strict skin dose constraints and implementing timely supportive care.
Adaptive radiotherapy was essential in this case. The notable 69% reduction in nodal GTV volume during treatment could have shifted nearby OARs into high-dose areas, increasing the risk of toxicity. Replanning mid-course enabled improved target coverage and better sparing of sensitive structures such as the skin and pharyngeal constrictors. However, such adaptive approaches require substantial institutional resources, including additional imaging, recontouring, and reoptimization capacity, which may be challenging in high-throughput clinical settings. Reference Castelli, Simon, Lafond, Perichon, Rigaud, Chajon, De Bari, Ozsahin, Bourhis and de Crevoisier9
Additionally, the risk of late vascular complications, particularly involving the carotid arteries, must be carefully considered. In this case, the carotid dose was held below 67 Gy, even if the vessel was in very close proximity to the tumor, aligning with growing evidence on the importance of carotid-sparing techniques in long-term survivors of head and neck cancer. Reference Leboucher and Sotton10
Conclusion
This case supports the feasibility and potential utility of VMAT with central GTV dose escalation using SIB in managing bulky nodal disease in p16-negative oropharyngeal SCC, particularly in patients who are not candidates for concurrent chemo-radiotherapy. The approach facilitated selective intensification of radiation dose to radioresistant tumor subregions while maintaining functional outcomes and minimizing toxicity. Mid-treatment adaptive replanning further enhanced treatment precision in response to tumor regression. While this technique represents a practical and resource-efficient alternative to biologically guided dose painting, additional studies are warranted to confirm its efficacy and define patient selection criteria in broader clinical settings.
Competing Interests
None.
Funding Statement
None.







