Introduction
Radiotherapy is an important modality in head and neck (H&N) cancer management, particularly where preservation of organ function is of concern.Reference Argiris, Karamouzis, Raben and Ferris1, Reference Bhide and Nutting2 Technological advances, such as intensity modulated radiotherapy, have allowed for dose escalation to the target volume and improved conformity, resulting in better outcomes and reduced treatment related toxicity.Reference Bhide and Nutting2 Volumetric-modulated arc therapy (VMAT) utilises the rotational potential of the linear accelerator to deliver highly conformal treatments through an arc of 360°.Reference Otto3, Reference Slotman, Doornaert, Bieker and Senan4
Changes to patient contour can impact upon the dose across the planning target volume (PTV), dose to organs at risk (OARs), or both; potentially to the extent that the plan requires modification.Reference Hansen, Xia, Quivey, Weinberg and Bucci5, Reference Barker, Jerry and Garden6 There is evidence that VMAT plans are more susceptible to these changes,Reference Winkler, Lodron and Mayer7 and are more complex to amend, than three-dimensional conformal radiotherapy (3D-CRT).Reference Brown, Porceddu, Owen and Harden8, Reference Court, Tishler, Petit, Cormack and Chin9
Image guided radiotherapy is increasingly employed as the steep isodoses of VMAT, combined with the proximity of OARs in the H&N, makes the risk and consequences of geographical miss increasingly severe.Reference Bhide and Nutting2 Alongside improved targeting, the use of cone-beam computed tomography (CBCT) as an imaging modality allows for monitoring and assessment of morphological changes to the patient.Reference Moinuddin, Kilkenny and Davies10
There are two main factors effecting patient shape and contour; first, over a standard course of radiotherapy, tumour shrinkage is a possibility.Reference Hansen, Xia, Quivey, Weinberg and Bucci5 This is most apparent for virally mediated tumours such as human papilloma virus-positive squamous cell carcinoma (SCC). These tumours often present with bulky nodal disease and respond well to treatment showing a significant reduction in size as treatment progresses.Reference Brown, Porceddu, Owen and Harden8
The second factor is weight loss, as treatment progresses, related toxicities can impact upon the dietetic health of patients, which needs to be monitored to ensure patient health is maintained.Reference Garg, Yoo and Winquist11, Reference Donovan and Glackin12 Unintentional weight loss is an indicator of malnutrition13 and is associated with longer recovery from treatment related toxicity and reduced quality of life.Reference Ravasco, Monteiro-Grillo, Marques Vidal and Camilo14–Reference Head, Heitz and Keeney16 Furthermore, studies have suggested that malnutrition reduces overall survival.Reference Nitenberg and Raynard17, Reference Datema, Ferrier and Baatenburg de Jong18
Since November 2012, the implementation of VMAT at the audit department has seen 3D-CRT for H&N cancer become obsolete and anecdotal evidence suggests that the timing, pattern and severity of side effects have changed in conjunction with the application of the VMAT technique. Identification of patients who are likely to experience weight loss can help to guide preventative action. It was the aim of this project to investigate the weight loss experienced by H&N cancer patients receiving VMAT; and whether any predictive factors can be identified.
Materials and Methods
This study formed part of a larger departmental audit that is investigating the impact of radiotherapy on H&N cancer patients. All data were anonymised and permission was sought and granted to carry out the audit from the Trust and data collection was in accordance with its guidelines.
Data collection
Data were gathered from the medical records of patients who had undergone radiotherapy treatment at the audit department. Eligibility criteria were: diagnosis of H&N cancer, treatment with curative intent using VMAT and weight monitored throughout radiotherapy. Patients were selected semi-randomly to account for gender. A sample size of 40 was used to meet time constraints while remaining representative of the patient group. Data for the first five patients were collected by two independent researchers, thereafter, every tenth patient was independently verified for consistency and to eliminate researcher bias.
Treatment
RapidArc treatments were generated in eclipse using AAA planning algorithm and delivered using Varian Clinac linear accelerators (6 MV photons) with CBCT capabilities. Patients received 60–70 Gy in 30–35 fractions. Induction chemotherapy was up to three cycles of Docetaxel, Cisplatin and 5FU. Concurrent chemoradiotherapy was up to three cycles of Cisplatin in weeks 1, 4 and 7 of radiotherapy. One patient received eight weekly infusions of Cetuximab starting 1 week before radiotherapy.
Departmental protocol states that patients should be weighed on the first day of treatment, once weekly thereafter and referred to dietetic services for weekly review. Patients who lose ≥10% of their body weight are admitted as an inpatient for closer monitoring and management of their condition.
An unintended reduction in body weight >5% in 3 months is considered by some as suggestive of malnutrition,19 NICE13 have suggested >5% in 3–6 months, while others studies have used >5% in 1 month, or >10% in 6 months.Reference van den Berg, Rasmussen-Conrad, van Nispen, van Binsbergen and Merkx15 Langius et al.Reference Langius, Doornaert, Spreeuwenberg, Langendijk, Leemans, Schueren and Marian20 considers unintentional weight loss >5% during a course of radiotherapy as risking malnutrition, as have othersReference Ehrsson, Langius-Eklöf and Laurell21, Reference Mallick, Gupta and Ray22; therefore, the same value has been applied within this work.
Analysis
Data were analysed using SPSS version 21. Descriptive statistics were used to identify variables with differences/trends in weight loss. Correlation was tested using Pearson’s correlation coefficient. Owing to the small sample size when distributed among nominal variables, non-parametric tests were used to test for significance. Multiple linear regression was then used to identify predictive factors for weight loss from factors individually identified as significant (p=0·05). Initially all variables were inserted, those identified as significant were then entered into the second analysis first with the remaining variables (not significant upon first analysis) entered one at a time to test for improved predictive power.
Results
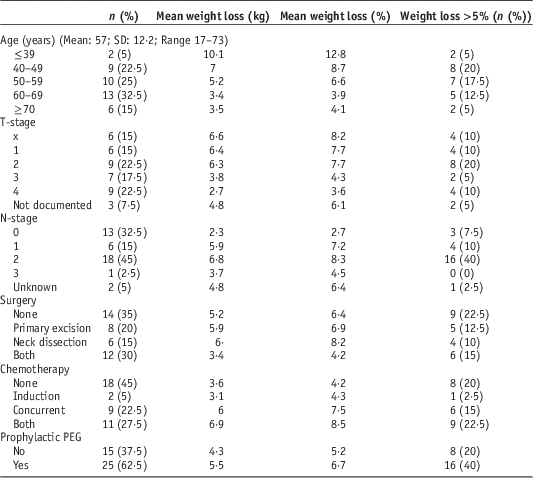
Descriptive statistics of the cohort are presented in Table 1. Data were obtained for 22 male and 18 female patients. Mean age was 56·5 years (M=58·4; F=54·3). A total of 29 (72·5%) patients were diagnosed with SCC, the commonest site was oropharynx (9/22·5%), followed by oral cavity (7/17·5%). A total of 13 (32·5%) patients were node negative; 26 (65%) patients underwent surgery, 22 (55%) received chemotherapy and 25 (62·5%) received prophylactic percutaneous endoscopic gastrostomy tubes (PEG). One patient struggled to eat and lost weight post-surgery and one patient reported 3–4 months of unintended weight loss before diagnosis. A total of 28 patients had weight measurements for every week of treatment, the remainder had 1 or more weeks missing.
Table 1 Cohort descriptives

Abbreviation: PEG, percutaneous endoscopic gastrostomy.
The range of weight loss was from 1 kg (1·8%) gained to 13·4 kg (15·9%) lost (Figure 1). The mean change was a reduction of 5·0 kg (SD=3·7)/6% (SD=4·4). Three (7·5%) patients gained weight throughout radiotherapy, 14 (35%) lost ≤5%, 17 (42·5%) lost >5% and ≤10%, and six (15%) lost >10%.

Figure 1 Range of weight loss.
Risk factors
Age, T-stage, nodal status, start weight and radiotherapy dose were tested for correlation with weight loss. Age and T-stage were negatively correlated, while nodal status and dose were positively correlated. Two patients were considerably younger (F: 17 and M: 28 years) than the next youngest (43 years) and the mean (57 years); and both suffered severe weight loss (9·2 and 11 kg; 14·2 and 11·4%, respectively). Correlation was recalculated without these data and while the correlation coefficient reduced it was still significant (p=0·001). Start weight approached, but did not achieve significance, for absolute weight loss.
Independent paired samples were tested using the Kolmogorov–Smirnov Z test. There was a significant increase in median % weight loss for patients receiving either concurrent, or induction and concurrent chemotherapy. Receipt of any chemotherapy versus none approached significance for both absolute and percentage weight loss.
For nominal variables with several categories the Kruskal–Wallis one-way analysis of variance was used. Chemotherapy (none versus induction versus concurrent versus both) and site both approached significance for absolute weight loss. Percentage weight loss showed significant differences according to chemotherapy regime (p=0·031). Patients receiving combined induction and concurrent chemotherapy demonstrated significantly more weight loss than those who did not receive any (p=0·033). Site approached significance for percentage weight loss.
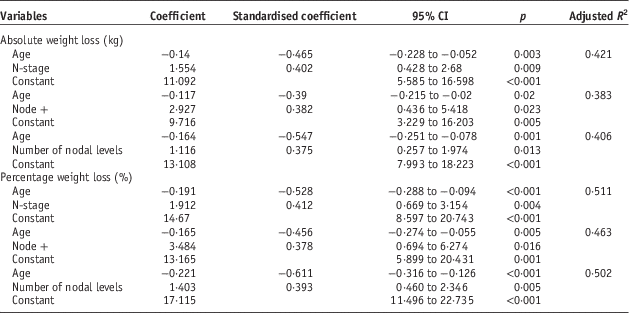
Multiple linear regression analysis was used to identify predictive factors using the variables identified as significant. To avoid co-linearity separate analyses were run for chemotherapy regimes. For both absolute and percentage weight loss, age and nodal status were the only predictive factors (Table 2). All combinations of categories were tested and each provided the same outcome regarding significant predictive factors.
Table 2 Multiple regression for absolute and percentage weight loss

Discussion
Weight loss has long been recognised as a problemReference Johnston, Keane and Prudo23; and remains a clinically significant complication of H&N radiotherapy.
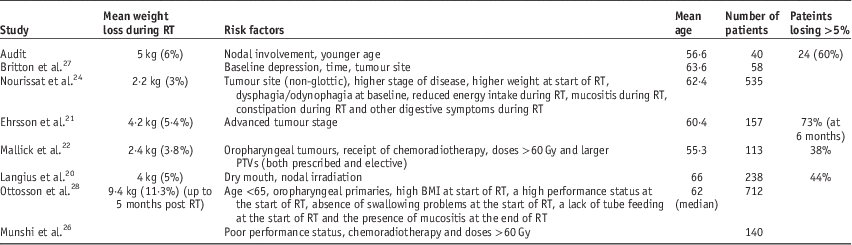
Not all patients will lose weight, but many do, with some having experienced some degree of weight loss as either symptomatic of disease, or related to previous treatment interventions.Reference Nourissat, Bairati and Fortin24, Reference Capuano, Gentile, Bianciardi, Tosti, Palladino and Di Palma25 In this study mean weight loss was similar, although at the top end of the range reported in other studiesReference Langius, Doornaert, Spreeuwenberg, Langendijk, Leemans, Schueren and Marian20, Reference Ehrsson, Langius-Eklöf and Laurell21, Reference Nourissat, Bairati and Fortin24 (Table 3). A total of 60% (24) patients had severe weight loss (>5%), which is higher than that reported at the end of radiotherapy in other studiesReference Langius, Doornaert, Spreeuwenberg, Langendijk, Leemans, Schueren and Marian20, Reference Mallick, Gupta and Ray22; 15% (six) patients lost >10% body weight throughout radiotherapy, fewer than the 74·2% reported by Munshi et al.,Reference Munshi, Pandey, Durga, Pandey, Bahadur and Mohanti26 but similar to Mallick et al.Reference Mallick, Gupta and Ray22 and Langius et al.Reference Langius, Doornaert, Spreeuwenberg, Langendijk, Leemans, Schueren and Marian20
Table 3 Key points

Abbreviations: RT, radiotherapy; PTV, planning target volume; BMI, body mass index.
Final weight measurements were taken during the last week of radiotherapy (in some cases earlier if final week data were not available). Mallick et al.Reference Mallick, Gupta and Ray22 studied up to 1 month post-radiotherapy; 52 out of 103 patients had data for this time point. Mean weight loss was higher than at the end of radiotherapy (4·1 versus 3·8%) but rates of weight loss >5% were similar (36·5 versus 37·9%). Nutritional scores in Britton et al.Reference Britton, Clover and Bateman27 were at their worst at the end of radiotherapy and improved by 4 weeks post-radiotherapy. Ehrsson et al.Reference Ehrsson, Langius-Eklöf and Laurell21 and Otosson et al.Reference Ottosson, Zackrisson, Kjellén, Nilsson and Laurell28 conducted longer follow-ups and found that weight loss peaked at 6 and 5 months, respectively. Langius et al.Reference Langius, Doornaert, Spreeuwenberg, Langendijk, Leemans, Schueren and Marian20 had data at 2 years post-radiotherapy for malnourished (weight loss >5%) patients (44% of the cohort), 73% of whom had not recovered to baseline weight. It seems reasonable to expect that patients in this cohort would have continued losing weight in the weeks and months following radiotherapy.
Linear regression showed nodal status and age to be predictive of weight loss. Correlations were stronger for percentage over absolute weight loss. Percentage weight loss adjusts for differences in start weight, which can be significant, particularly between genders.
Treatment characteristics
Chemotherapy and radiotherapy doses were associated with increased weight loss in this study, but neither was predictive. Previous studies have found chemoradiotherapy and radiotherapy doses >60 Gy were both associated with increased weight loss.Reference Mallick, Gupta and Ray22, Reference Munshi, Pandey, Durga, Pandey, Bahadur and Mohanti26 Concurrent chemotherapy and higher doses of radiotherapy are known to increase toxicity, potentially leading to dysphagia, reducing capacity for oral intake.Reference Platteaux, Dirix, Dejaeger and Nuyts29 The associated risk of weight loss is therefore understandable. Interestingly, the cohort studied by Langius et al.Reference Langius, Doornaert, Spreeuwenberg, Langendijk, Leemans, Schueren and Marian20 did not receive chemotherapy, yet still showed proportionally similar weight loss. As radiotherapy doses are unlikely to be reduced, patients receiving the higher doses may require closer surveillance and additional support in attempts to minimise weight loss.
Patient characteristics
Munshi et al.Reference Munshi, Pandey, Durga, Pandey, Bahadur and Mohanti26 identified poor performance status (PS) to be associated with increased weight loss, whereas Ottosson et al.Reference Ottosson, Zackrisson, Kjellén, Nilsson and Laurell28 found the opposite. Theoretically, patients with lower PS would do worse; however, if these patients are identified before treatment as at risk, early intervention may minimise weight loss. PS may reflect the preferences of the centre studied rather than any patient or treatment related characteristic; although it should be noted that Ottosson et al.Reference Ottosson, Zackrisson, Kjellén, Nilsson and Laurell28 was a multicentric study.
Younger patients lost more weight in this study and age was predictive, while other studies have also observed increased weight loss for younger patients,Reference Langius, Doornaert, Spreeuwenberg, Langendijk, Leemans, Schueren and Marian20, Reference Ottosson, Zackrisson, Kjellén, Nilsson and Laurell28 although neither were predictive and others have found no association with age.Reference Ehrsson, Langius-Eklöf and Laurell21 Despite the latter, there appears to be a trend of younger patients losing more weight, possibly associated with more aggressive treatments, or psychological impact. Further study in this area is required to understand causal relationships and potential corrective interventions, whether they be nutritional, or psychological.
Start weight has been found to be weakly associated, but predictive of weight loss.Reference Nourissat, Bairati and Fortin24 Another study used body mass index measures, which showed significant differences in weight loss between overweight/obese and normal/underweight patients.Reference Ottosson, Zackrisson, Kjellén, Nilsson and Laurell28 Percentage weight loss accounts for differences in start weight at baseline. If weight loss is used as indicative of malnutrition, relative values are more reliable as the clinical relevance of a 5 kg reduction can vary according to the patient’s start weight.
Other identified risk factors for increased weight loss include the presence of dysphagia at baselineReference Nourissat, Bairati and Fortin24 and no swallowing problems at baselineReference Ottosson, Zackrisson, Kjellén, Nilsson and Laurell28 reduced energy intake throughout radiotherapy, the presence of digestive symptoms during radiotherapy,Reference Nourissat, Bairati and Fortin24 xerostomiaReference Langius, Doornaert, Spreeuwenberg, Langendijk, Leemans, Schueren and Marian20 and mucositis during radiotherapy.Reference Nourissat, Bairati and Fortin24, Reference Ottosson, Zackrisson, Kjellén, Nilsson and Laurell28 Mucositis and xerostomia are notable factors that potentially limit oral intake, perhaps explaining reduced intake.
Studies have largely focussed on clinical factors, and easily defined patient characteristics such as age, cohabitation or smoking status. Only one study was found that investigated psychological factors; showing depression as predictive of weight loss, a factor considered potentially modifiable, offering a new avenue for minimising weight loss.Reference Britton, Clover and Bateman27 The lack of study in this area also suggests that depression may be an aspect of cancer care that is not being fully addressed.
Disease characteristics
This audit found nodal status to be predictive of weight loss, while Mallick et al.Reference Mallick, Gupta and Ray22 found that larger PTVs were predictive. In the study by Langius et al.Reference Langius, Doornaert, Spreeuwenberg, Langendijk, Leemans, Schueren and Marian20 irradiation of neck nodes was the most reliable factor for predicting significant weight loss. Node positive patients and those at risk of nodal involvement require larger PTVs to encompass the volume designated for radical or elective irradiation. Consequently, the volume of tissue included in the high dose region is greater, increasing the severity and extent of toxicities. This could help to explain the higher risk of weight loss, either through increased metabolic demands or reduced oral capacity.
Mallick et al.Reference Mallick, Gupta and Ray22 also analysed N-stage, comparing N0/1 versus N2/3, which had no associated difference. N1 patients would ordinarily receive a radical dose to involved nodes and an elective dose to the next level nodes; whereas N0 patients may have no nodal irradiation, depending on risk factors. Combining N0-1 may hide differences between node positive and node negative patients. Nodal status or PTV volume, strongly suggest that radiotherapy related toxicity is an important causal factor in weight loss and could be used to reliably indicate patients at risk.
Here, T-stage was negatively correlated with weight loss, whereas other studies have shown weight loss to increase with stage.Reference Langius, Doornaert, Spreeuwenberg, Langendijk, Leemans, Schueren and Marian20, Reference Ehrsson, Langius-Eklöf and Laurell21 Positive correlation between stage and weight loss is more intuitive as the expected impact of more advanced disease requiring wider treatment fields would be increased toxicity for these patients. This perhaps indicates that higher stage patients at the study centre are identified as at risk and managed proactively; or that they are considered less able to tolerate intense treatment and thus suffered less severe toxicities.
Differences were observed between primary sites, although were not significant upon analysis. Some studies have shown that oropharyngeal tumours were predictive of increased weight lossReference Mallick, Gupta and Ray22, Reference Ottosson, Zackrisson, Kjellén, Nilsson and Laurell28 and others with non-glottic tumour showed increased weight loss.Reference Nourissat, Bairati and Fortin24 However, Langius et al.Reference Langius, Doornaert, Spreeuwenberg, Langendijk, Leemans, Schueren and Marian20 only studied early stage laryngeal patients who displayed similar rates of weight loss to studies of heterogeneous primary site. Primary site has been identified as a factor in previous studies, but if taken as a sole predictor leaves many patients at risk, impairing both the quality of treatment and support received. Discomfort from treatment related toxicity of swallowing structures can help explain this trend,Reference Murphy and Gilbert30 as can a weakening of musculature associated with swallowing if included in treatment fields.Reference Deantonio, Masini, Brambilla, Pia and Krengli31
Enteral feeding
The discrepancy noted above regarding the presence of swallowing problems seems indicative of patient management. In Ottosson et al.,Reference Ottosson, Zackrisson, Kjellén, Nilsson and Laurell28 76 (10%) patients received enteral nutrition from the start of radiotherapy, this included patients with swallowing problems. That figure increased to 335 (47%) patients by the end of radiotherapy. By contrast only 14 (3%) patients received enteral nutrition in Nourissat et al.,Reference Nourissat, Bairati and Fortin24 whereas 29 (6%) patients started radiotherapy with oral supplements, rising to 275 (51%) during radiotherapy.
There is no consensus on prophylactic enteral feeding as evidence shows both positive and negative outcomes. In the audit department prophylactic PEGs are offered only to patients undergoing chemoradiation.
It has been suggested that enteral feeding helps to prevent malnutrition,Reference Ottosson, Zackrisson, Kjellén, Nilsson and Laurell28 as has been demonstrated in numerous studies.Reference Beaver, Matheny, Roberts and Myers32–Reference Assenat, Thezenas and Flori36 The European Society for Clinical Nutrition and Metabolism recommend the use of enteral feeding where prolonged reduced nutritional intake is anticipated, for example, through obstruction or treatment related side effects.Reference Arends, Bodoky and Bozzetti37 However, enteral feeding has been linked with increased dysphagia, potentially explained by reduced exercise of the swallowing musculature.Reference Langmore, Krisciunas, Miloro, Evans and Cheng38–Reference Stuart, Luu and Farwell40 Prophylactic feeding tubes may encourage cessation of oral intake earlier than would otherwise be the case, increasing tube dependency.Reference Oozeer, Corsar, Glore, Penney, Patterson and Paleri41
In Ottosson et al.Reference Ottosson, Zackrisson, Kjellén, Nilsson and Laurell28 patients without prophylactic enteral feeding exhibited increased weight loss, whereas the study by Ehrsson et al.Reference Ehrsson, Langius-Eklöf and Laurell21 showed patients receiving enteral nutrition were associated with increased weight loss. In the latter three (2%) patients received prophylactic enteral nutrition while 89 (56%) started during or after completion of radiotherapy. Enteral nutritional intervention was predominantly reactive in both studies, and therefore, is not predictive. These studies suggest that reactive enteral nutrition alone is not effective in combatting weight loss. This study observed a trend for increased weight loss in the PEG group but this did not obtain significance.
Weight loss and malnutrition are prevalent in H&N patients before, during and after radiotherapy.Reference Langius, Doornaert, Spreeuwenberg, Langendijk, Leemans, Schueren and Marian20, Reference Ehrsson, Langius-Eklöf and Laurell21, Reference Ottosson, Zackrisson, Kjellén, Nilsson and Laurell28 Potential predictive factors for weight loss were identified in this study. Several other studies have been carried out with similar aims, most have been retrospective and as yet no clear model has emerged. Identified risk factors include primary tumour site, high starting weight, extent of dysphagia, chemoradiotherapy, radiotherapy dose, PS, stage of disease and depression.Reference Ehrsson, Langius-Eklöf and Laurell21, Reference Mallick, Gupta and Ray22, Reference Munshi, Pandey, Durga, Pandey, Bahadur and Mohanti26–Reference Ottosson, Zackrisson, Kjellén, Nilsson and Laurell28 The full aetiology of weight loss is not yet known, nor is the extent of risk factors fully understood. Studies often appear contradictory, as they can reflect how patients are managed at different centres as much as they identify genuine risk factors for weight loss. This study emphasises the requirement for nutritional support for H&N cancer patients and offers some insight into factors predicting for weight loss.
Study limitations
A small sample limited the power of the study. Data were collected entirely from patient notes of those who had completed treatment restricting the range of variables that could be tested. Data were not available for every patient in every variable, effectively reducing the sample size for those variables.
Nonetheless, this study can help to inform future practice. Weight loss remains a problem for H&N patients. Nodal status and age were identified as predictive factors and both have been identified in other studies helping to verify their significance.Reference Langius, Doornaert, Spreeuwenberg, Langendijk, Leemans, Schueren and Marian20, Reference Ottosson, Zackrisson, Kjellén, Nilsson and Laurell28 Factors identified by other studies, such as site, or PS were not so hereReference Munshi, Pandey, Durga, Pandey, Bahadur and Mohanti26, Reference Ottosson, Zackrisson, Kjellén, Nilsson and Laurell28; substantiating claims that weight loss is complex and multifactorial.Reference Britton, Clover and Bateman27 As a guideline for clinical use age and N-stage are readily available and could be informative in assessing a patient’s risk of weight loss.
Implications for practice
Further investigation is required to better understand this phenomenon. Prospective study may help to clarify risk factors and could be augmented with self-report questionnaires and a larger sample. Ultimately more robust study will provide opportunity to develop a clinical model for predicting weight loss and malnutrition; allowing for assessment, identification and stratification of patients at risk of weight loss.
Conclusion
Weight loss is common among H&N cancer patients. In recent years attempts have been made to identify causes and risk factors. In this study weight loss was typical, with 60% patients losing >5% of their starting body weight. Age and nodal status were identified as predictive factors in this cohort. The reasons behind weight loss are likely to be complex and multifactorial. The need for further study is two-fold:
1. To better understand weight loss, enabling early identification of at risk patients and to provide the required level of nutritional support.
2. To evaluate the effectiveness of nutritional interventions to understand what nutritional support should entail.
Acknowledgement
With thanks to Sheffield Teaching Hospitals NHS Trust for granting permission for this project to be carried out.
Financial Support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflicts of Interest
None.