Introduction
Evidence is emerging to suggest that intensity modulated radiation therapy (IMRT) is clinically superior to three-dimensional conformal therapy (3D-CRT).Reference Veldeman, Madani, Hulstaert, De Meerleer, Mareel and De Neve1 IMRT can take longer to deliver than conformal therapy (3D-CRT), the time being dependent on planning parameters such as the number of beams or segments.Reference Kuperman, Ventura and Sommerfeldt2 Extending the delivery time may result in increased intra-fraction motion and potential loss of radiobiological effectiveness.Reference Bewes, Suchowerska, Jackson, Zhang and McKenzie3 Extended delivery times inherent in IMRT delivery compared with 3D-CRT will also result in economic differences as more time will be required on the linear accelerator for both treatment and quality assurance. There is increasing evidence that volumetric modulated arc therapy (VMAT) techniques can create equivalent or better treatment plans, which can be delivered within a much faster timeframe compared with IMRT plans.Reference Zhang, Happersett, Hunt, Jackson, Zelefsky and Mageras4–Reference Bzdusek, Friberger, Eriksson, Hårdemark, Robinson and Kaus8
By assuming that the machine dose rate can vary as required, OttoReference Otto9 developed a single-arc algorithm referred to as VMAT.Reference Yu and Tang10 Rapidarc® plans can utilise the latest hardware and software control systems on Varian linacs to allow continuously variable dose-rate (vdr) VMAT delivery. Upgrades in both hardware and software are available for some, but not all, existing treatment machines, however, such upgrades come at a financial cost. Therefore, a large number of existing Varian linacs can only deliver rotational arcs [while varying the multileaf collimator (MLC) positions] using a constant dose-rate (cdrVMAT) delivery. A methodology has been proposed to convert variable dose rate plans to constant dose rate plans without degradation of plan quality at the expense of treatment time in most cases.Reference Tang, Earl and Yu11 The authors acknowledged that ideally constant dose rate plans should be prospectively planned to reduce planning time. However, using the predecessor to Rapidarc® it was shown that planning using cdrVMAT plans were inferior for prostate patients compared with IMRT five-field plans.Reference Palma, Vollans and James12 The difference in delivery time or accuracy was not assessed in this study.
It has been shown that it is possible to deliver cdrVMAT plans using a multi-vendor environmentReference Feygelman, Zhang and Stevens13 using an IMRT test suite.Reference Ezzell, Burmeister and Dogan14 However, no study has investigated both the planning and delivery characteristics of prostate patients using cdrVMAT compared with five-field IMRT. Feygelman et al.Reference Feygelman, Zhang and Stevens13 created plans using 360° arcs and stated that Pinnacle™ does not correct the calculated dose for treatment couch attenuation. A correction was made using a single factor, post planning to account for the image guided radiation therapy (IGRT) couch, which is designed to minimise beam attenuation for kV imaging. It has been shown that a maximum dose error of 2·1% can occur at the planning target volume (PTV) if this couch is not taken into account.Reference Vanetti, Nicolini, Clivio, Fogliata and Cozzi15 Indeed Li et al.Reference Li, Lee, Johnson, Zhu and Kudchadker16 showed that the IGRT couch can attenuate by up to 4·1% for oblique angles and this increased to 28·1% for a standard (non-IGRT) couch.
Many other types of couch may be used with different couch attenuation properties. Largest attenuation is at gantry angles of 110–120° with a reduction until the beam becomes perpendicular at 180° (posterior).Reference Seppälä and Kulmala17, Reference Smith, Christophides, Dean, Naisbit, Mason and Morgan18 There is a possibility to write script extensions into treatment planning systems such as Pinnacle™ although this is not possible with all treatment planning systems and the expertise is not available in every department. Using a treatment planning system which models the couch, Pulliam et al.Reference Pulliam, Howell, Followill, Luo, White and Kry19 showed that not including the couch top and rails within the plan could reduce the tumour control probability (TCP) by up to 10·5%. Not all treatment planning systems incorporate the couch top and rails into the treatment planning system,Reference McGarry, O'Sullivan and Hounsell20 which can result in a significant reduction in dosimetric accuracy. A method can be applied where the couch can be outlined in the plan and fused with the external body contour although this would become cumbersome for a large number of patients. Inherent modelling of the couch within the treatment planning system is an efficient solution to this problemReference Pulliam, Howell, Followill, Luo, White and Kry19 although another potential solution is avoidance of the couch.
IMRT and VMAT plans are typically inverse planned using an optimisation algorithm, which can vary between different in-house and commercial solutions. Comparing radiotherapy treatment plans can be compromised by the fact that there are competing trade-offs such as the target uniformity against dose to a critical structure. Pareto fronts have emerged as a tool for comparing treatment plans as it gives better visualisation of the trade-offs.Reference Ottosson, Engstrom and Sjöström21–Reference Craft, Halabi and Bortfeld23 A Pareto optimal treatment plan is one for which there does not exist another plan that is strictly better in at least one objective function while being no worse in every other objective. A situation can exist whereby non-clinically acceptable plans may need to be included to create the entire curve when two competing variables are plotted (typically target and critical structure). During the optimisation process, despite protocols, the final choice of the correct solution will be planner dependent. However, in comparing Pareto fronts for different techniques, a bias towards planner experience can be minimised.
The aim of this work is to create cdrVMAT plans which avoid the requirement for a couch model and assess the dosimetric and delivery characteristics of the plans compared with IMRT plans. A method is presented whereby the clinical acceptability of the plans is shown on the Pareto curve following comparison of cdrVMAT plans with IMRT plans. We also show the radiobiological impact of these curves. Clinically acceptable plans with the lowest average rectal dose are compared between different techniques and delivery methods.
Method
Treatment planning
All treatment planning was performed on a single PC (HP z8000 Workstation, two Intel® Xeon® CPU X5660 2·8 GHz and 12 Gb of RAM) using the Oncentra® (Nucletron BV, Veenendal, the Netherlands) treatment planning system v4.1 (Nucletron BV, Veenendal, the Netherlands). The pencil beam dose calculation algorithm was used for both IMRT and VMAT plans. For both algorithms the enhanced version was utilised which includes modelling of the MLC type, MLC transmission and source size. Treatment plans were designed for delivery on a Varian 2100CD linear accelerator equipped with 120-leaf millennium MLC (Varian Medical Systems, Palo Alto, CA, USA) via the ARIA record and verify system, using 6 MV photons.
Plans were created using computed tomography (CT) datasets of ten successive prostate cancer patients (Ethics REC ref 09/NIR02/28). Each patient had been CT scanned using a GE Lightspeed wide-bore scanner (GE Healthcare, Waukesha, WI, USA) at 2·5 mm slices. Target volumes were expanded and organs at risk delineated, including rectum, bladder, femoral heads, penile bulb and bowel, according to the CHHiP protocol.Reference Khoo and Dearnaley24 Plans were created with a prescription of 60 Gy in 20 fractions. Dose levels to the targets and tolerances for organs at risk are shown in Table 1. Figure 1 shows axial views of all ten patients including the targets and rectum at isocentre. An avoidance shell was delineated which ensured a steep fall-off of dose from the targets to surrounding area. This shell was designed for the purpose of this study to not involve the rectum.
Table 1 Selection of dosimetric quality parameters outlined in the CHHIP trial protocol

Notes: PTV min is the minimum dose (%) received by 99% of the volume. For critical structures, the constraint is the maximum dose received by the volume X where v(X)max is shown (e.g., – X = 68 for femoral heads).
Abbreviation: PTV, planning target volume.

Figure 1 Depiction of the three targets with the rectum (turquoise) for all ten patients including PTV 1 (blue), PTV 2 (green) and PTV 3 (purple).
IMRT plans were created using direct step and shoot and delivered using the step and shoot method with a dose rate of 400 MU/min. A five-field class solution was used with beam angles of 35°, 100°, 180°, 260° and 325°. These beam angles were used so as to minimise couch attenuation and also delivery time. This class solution has been developed from beam angles published previously.Reference Mott, Livsey and Logue25 The number of segments was 50 per plan, the minimum field size was 4 cm2 and the grid size was set to 0·25 cm in all directions.
All VMAT plans used a calculation grid size of 0·35 cm in each direction as this was the minimum allowed using a typical quad core computer for calculation of a treatment plan. VMAT optimisation in Oncentra® is similar to that reported for the version later implemented in Pinnacle Smartarc®.Reference Bzdusek, Friberger, Eriksson, Hårdemark, Robinson and Kaus8 Coarse initial control points with fluence maps are generated at the start and at 24° increments until the stop angle. Intensity modulation is performed resulting in an ideal fluence and this is converted to 2–4°-spaced control points. The smallest MLC openings are filtered out leaving two control points per initial angle. These control points are distributed to adjacent gantry angles and a sorting algorithm determines the order of the control points to minimise leaf travel. Additional control points are added through a cloning method.
VMAT plans started at 260° and rotated 200° clockwise with 4° gantry spacing with a second counter-clockwise arc rotating at 200° with 4° spacing resulting in the gantry finishing position at the original 260°. The maximum delivery time set was 45 seconds per arc following initial analysis on a single patient. For one patient this was increased to 60 seconds per arc as no plans were acceptable with 45 seconds. The minimum and maximum numbers of monitor units (MU) per gantry angle were set at 0·1 and 20·0, respectively. Maximum leaf speed was set at 1·5 cm/s with static and dynamic leaf gaps set at 0·05 and 0·10 cm, respectively. For cdrVMAT plans the dose rate was fixed during the delivery and was selected from a discrete table of 100, 200, 300, 400, 500 or 600 MU/minute. The collimator was set to 45° for all VMAT plans to reduce the cumulative effects of interleaf transmission and the tongue and groove effect.
All plans were assessed in terms of acceptability according to the CHHiP protocol.Reference Khoo and Dearnaley24 Each of the indices specified in Table 1 were also compared across all plans. Further analysis was performed on targets in the form of homogeneity index (HI) to PTV 3 defined as D5%–D95% (where D5 is the dose to 5% volume and D95 is dose to 95% volume) and conformity index (CI) calculated as the volume of the body receiving the prescribed dose divided by the corresponding PTV. The irradiated whole body volume has been reported using a number of different indices including >v5 GyReference Boylan, Golby and Rowbottom26 and >v20%.Reference Bedford6 The absolute whole body volume irradiated to 10%, 20% and 40% of the prescribed dose were recorded.
Pareto front generation
A plan is Pareto optimal if it is impossible to improve one aspect without worsening another. In reality, it is seldom known whether the treatment plan resulting from the optimisation routine of a commercial treatment planning system is Pareto optimal. If one can accept the noise on fronts because of uncertainty and feel that this has little practical consequence, then the set of deliverable pseudo-optimal plans can combine to create a Pareto front.Reference Petersson, Ceberg, Engström, Benedek, Nilsson and Knöös22
An initial IMRT plan was designed to deliver the required doses to PTV 1, PTV 2 and PTV 3 with maximum uniformity with no objective or constraint on the rectum. Figure 2 shows that a shell was delineated around these targets excluding the rectum to ensure that the trade-off was between the target (PTV 3) and critical structure (rectum). An average dose to rectum constraint was added to the plan, which was re-optimised. A series of plans (eight or more) were calculated where the constraint reduced the dose to the rectum incrementally. This was repeated for the dual arc cdrVMAT plans. Each plan was assessed in terms of the CHHiP parameters for acceptability of dose-volume histogram (DVH) parameters and dose distributions. Pareto fronts were created comparing the minimum dose to the PTV 3 volume with the average dose to the rectum for each patient. Within the CHHiP protocolReference Khoo and Dearnaley24 the minimum dose is defined as the dose received by at least 99% of the volume D(99). This is similar to what should be reported according to ICRU 83.Reference Grégoire and Mackie27 To ensure that a Pareto curve would be created, 100−D(99) was plotted against the average dose. Within these plots, information on the acceptability of each plan was displayed.

Figure 2 Targets outlined to receive at least 76% (PTV 1), 91% (PTV 2) and 95% (PTV 3) of prescribed dose with avoidance of the rectum shown. A shell (red), not including the rectum was included to ensure conformity.
Biological modelling
Each DVH was also used to investigate the TCP and rectal normal tissue complication probability (NTCP). A Poisson TCP model was used.Reference Webb and Nahum28 This model is based on the number of cells killed per fraction, the clonogen density within the tumour, and incorporated a repair term to account for re-growth. Model parameters for prostate cancer were taken to be α = 0·15, α/β = 3·1, a clonogen density of 10,000 cells per cm3 for PTV 3 and a cell doubling time of 42 days.Reference Wang, Guerrero and Li29 TCPs were also calculated for the regions PTV 2–3 and PTV 1–2. Calculations for these regions used the same radiation response parameters and doubling time as PTV 3, but made use of a scaled clonogen density, based on the dose levels from the CHHiP protocol. However, as the clonogen density is purely a scaling factor in this analysis, the observed qualitative differences are independent of this choice. Rectal NTCP was calculated using the LKB ModelReference Kutcher, Burman, Brewster, Goitein and Mohan30 with parameters n = 0·12, m = 0·15 and TD50 = 80 Gy.Reference Burman, Kutcher, Emami and Goitein31
Planning and delivery analysis
A clinically acceptable plan for each patient with the lowest average rectal dose for each delivery type was used for further analysis. This allowed comparison between IMRT and cdrVMAT using the best possible plan generated using all planning and delivery parameters investigated.
The calculation times including optimisation and final dose calculation and the total number of MU per plan were recorded. Delivery times for each IMRT plan and cdrVMAT plan were recorded from the beam on time of the first field until the final beam-off time of the final field. Each clinically acceptable IMRT and cdrVMAT plan was recalculated on a CT scan-set of a MatriXX evolution 2D ionisation chamber array device (IBA, Schwarzenbruck, Germany) with 6-cm-thick 30 × 30 cm2 solid water above and below the device. All plans were delivered to the array using a Varian 2100CD linear accelerator and the measured data in the coronal plane was compared with that calculated using gamma analysis with the dose and distance criteria set at 3% and 3 mm, respectively. As a further check, the cdrVMAT plans were recalculated on a CT dataset and subsequently delivered to an ArcCheck diode array device (Sun Nuclear Corporation, Melbourne, FL, USA). Analysis used gamma analysis with the dose and distance criteria set at 3% and 3 mm, respectively.
The median and the first and third quartiles were presented for each parameter analysed for either IMRT or cdrVMAT. Statistical analysis was undertaken comparing the IMRT and cdrVMAT plans using Wilcoxon's signed rank-test with a level of significance placed at p = 0·05 (SPSS).
Results
Figure 3 shows Pareto fronts for ten patients comparing IMRT five-field plans and cdrVMAT plans. For the entire front it can be noted that the cdrVMAT points are lower than the IMRT points for patient 1, 2, 5, 7 and 9. For patient 3, 4, 6 and 10 no difference is observed between the two techniques across the entire Pareto front. An advantage of using IMRT was observed for patient 8. As the average rectal dose reduced, little effect was observed on clinical acceptability although once the HI gets poorer, there was an inclination for plans to fail. Other factors that contributed to failure were loss of coverage to PTV 1 or PTV 2. Further analysis plotting the bladder doses against rectal dose showed no trend for either IMRT or cdrVMAT above an average rectal dose of 25 Gy, which is where most clinically acceptable plans were, as shown in Figure 3. Below this rectal dose, the bladder dose tended to increase for the IMRT plans and decrease for the cdrVMAT plans.

Figure 3 Pareto fronts comparing intensity modulated radiation therapy (solid data-points) and cdrVMAT (open data-points) for patient 1–10. Plans shown are not acceptable (small circles), dose distributions only acceptable (downward triangles), DVH acceptable (upward triangles) or plan acceptable (large circles).
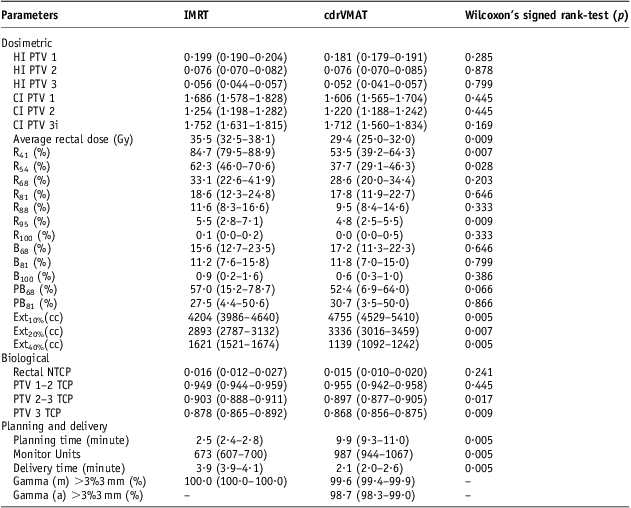
For each patient the clinically acceptable plan with lowest average rectal dose was selected for IMRT or cdrVMAT to allow a more statistically robust method of comparison. Table 2 summarises dosimetric data attained from the DVH of each IMRT and cdrVMAT plan for each patient. No statistical difference was observed between methods when analysing the HI or CI of all three PTVs. The average rectal dose was significantly less for the cdrVMAT plans than IMRT plans. Further assessment of the rectal DVH using dose points coincident with those with the tolerances shown in Table 1 revealed that less volume was irradiated to the 41–95% dose range using the cdrVMAT technique although this only appeared to be statistically significant at the 41%, 54% and 95% dose points. IMRT had less volume irradiated to 100% dose although this value was very small.
Table 2 Comparison of dosimetric, biological, planning and delivery parameters between five-field IMRT plans and cdrVMAT plans for ten prostate patients

Notes: Median, first and third quartiles. Wilcoxon's signed rank-tests performed for each with significance level at p = 0·05. Subscripts show percentage dose point that volume on DVH is analysed.
Abbreviations: IMRT, intensity modulated radiation therapy; cdrVMAT, constant dose-rate volumetric modulated arc therapy; PTV, planning target volume; HI, homogeneity index; CI, conformity index; R, Rectum; B, bladder; PB, penile bulb; Ext, External; NTCP, normal tissue complication probability; TCP, tumour control probability; m, MatriXX; a, ArcCheck.
The median rectal NTCP was lower for cdrVMAT but was not statistically significant. The TCP for the two main PTVs not including the seminal vesicles were statistically significantly lower for cdrVMAT plans. There is a ±1% tolerance on the prescription dose and further investigation showed that the mean dose was 99·9 ± 0·3% for cdrVMAT and 100·5 ± 0·3% for the IMRT plans. Therefore, the IMRT plans resulted in a larger TCP. For a single iteration cdrVMAT planning time took approximately four times as long as IMRT. MU were ∼53% more for cdrVMAT plans although this did not translate to longer delivery times as the plans were 42% faster to deliver than the IMRT plans. The cdrVMAT plans were shown to be delivered accurately with 99·2 ± 1·1% of pixels passing the gamma criterion of 3%3 mm although this accuracy was <100% of pixels passing for the IMRT plans using the MatriXX. The ten clinically acceptable cdrVMAT plans were also delivered to an ArcCheck device and had 98·6 ± 0·4% of pixels passing 3%3 mm.
Discussion
A method has been presented that allows planning and accurate delivery of VMAT plans to prostate patients using constant dose rate without the requirement for a couch model within the treatment planning system. The cdrVMAT plans were shown to be dosimetrically superior to IMRT although this did not translate into a biologically significant advantage. A distinct advantage in reduced delivery time by 45% was observed for the cdrVMAT plans. Pareto fronts, showing the stage at which plans became clinically acceptable were used.
This study has benefited from presenting the data as Pareto fronts as this minimised potential planning bias by showing all plans created. For 1/10 patients a distinct advantage was observed across the Pareto curve for cdrVMAT compared with IMRT with an overlay of the two curves for 9/10 patients. IMRT rather that cdrVMAT plans would have been more beneficial for one of the patients although it was difficult to find any acceptable plan using cdrVMAT for this particular patient. The main reason for failures against the protocol were the fact that the PTV 3 could not attain good coverage with lower rectal doses or that acceptable rectal doses for the given constraints (Table 1) could not be attained. Other reasons for failures were that the maximum dose of 105% was violated or the coverage of PTV 1 was not attained. It is interesting to note that the clinically acceptable plans were in a flat part of the Pareto front. Ultimately only a single plan can be used from the front to treat patients and the best plan was chosen as that with the minimum average rectal dose.
The biological analysis showed that 4/10 patients had a reduced NTCP for cdrVMAT with no distinct advantage observed for 5/10 patients and a single IMRT patient having lower NTCP. There was not a statistically significant difference in NTCP between the two techniques but Table 2 shows that there was a statistically significant reduction in TCP for PTV 3 and PTV 2 when using cdrVMAT. This reduced TCP is most likely related to the median dose to these targets, which have a tolerance of ±1% for PTV 3. For PTV 3 the median was 100·5±0·3% and 99·9 ± 0·3% for IMRT and cdrVMAT, respectively. Hence, the lower median dose has resulted in a lower TCP. Indeed Zhang et al.Reference Zhang, Happersett, Hunt, Jackson, Zelefsky and Mageras4 also observed a reduction in TCP for VMAT plans compared with IMRT plans due to the reduced mean dose to the target. This can be changed by increasing the dose to the target although the average rectal dose (and the NTCP) may increase.
The Pareto front presented for patient 8 in Figure 3 show the differences between IMRT and cdrVMAT and that the cdrVMAT plan could not easily be created as the coverage degraded quickly. Figure 1 shows that the planning targets for patient 8 are much more concave than the other patients with the largest overlap between targets and rectum. Therefore, it is clear that more complex anatomies may not benefit from the use of cdrVMAT using dual partial arcs. It may be that this patient would benefit from a 360° rotation or possibly a second complete arc. However, without a full couch model, delivery accuracy may be limited if the beams pass through the couch.Reference Vanetti, Nicolini, Clivio, Fogliata and Cozzi15, Reference Seppälä and Kulmala17, Reference Pulliam, Howell, Followill, Luo, White and Kry19
An increase in low dose whole body irradiation was observed for the cdrVMAT technique compared with IMRT. The magnitude of this increase was similar to that reported by other authors.Reference Zhang, Happersett, Hunt, Jackson, Zelefsky and Mageras4, Reference Bedford6, Reference Boylan, Golby and Rowbottom26 For the partial arc plans the low-dose region was different from complete arcs as there was no beam entry at points between 100° and 260° in the region where the couch was being avoided. The cdrVMAT whole body volumes irradiated to intermediate doses was less than those for IMRT.
More complex anatomies often require a second arc to produce similar or superior treatment plans to IMRT.Reference Bedford6, Reference Alvarez-Moret, Pohl, Koelbl and Dobler32 The reason for this may be limitations in current VMAT algorithms which create control points that are initially coarse followed by an interpolation of control points at the remaining gantry angles. This can result in poorer control on where to optimally modulate the gantry speed, although solutions are emerging.Reference Craft, McQuaid, Wala, Chen, Salari and Bortfeld33 Prostate IMRT treatments are typically less complex than other treatment sitesReference McGarry, Chinneck, O'Toole, O'Sullivan, Prise and Hounsell34 and therefore this is the most likely site that acceptable plans could be delivered without the added variable dose rate degree of freedom. Indeed Palma et al.Reference Palma, Vollans and James12 compared constant and variable dose rate plans and found constant dose rate plans to be inferior to both vdrVMAT and IMRT plans. Our work uses a different algorithm and different gantry rotations for plan creation resulting in properties, which were vastly different from those presented by Palma et al. (2008).Reference Palma, Vollans and James12 We found that more MU were required for cdrVMAT plans compared with IMRT plans, which is contrary to Palma et al.Reference Palma, Vollans and James12 Similar MU between VMAT and IMRT have been reported using this algorithmReference Dobler, Weidner and Koelbl5, Reference Alvarez-Moret, Pohl, Koelbl and Dobler32 although the linear accelerator used was an Elekta.
Despite an increased number of MU, Table 2 shows that the delivery time is significantly faster for the cdrVMAT plans compared with IMRT. This has not been previously reported. The difference in delivery time between IMRT and cdrVMAT is similar to previously reported reductions in delivery times using vdrVMAT.Reference Zhang, Happersett, Hunt, Jackson, Zelefsky and Mageras4, Reference Dobler, Weidner and Koelbl5, Reference Alvarez-Moret, Pohl, Koelbl and Dobler32 Faster delivery has the potential advantage of reduced intra-fraction motion and also has some economic aspects. Any disadvantage in going from conformal radiotherapy to IMRT in terms of delivery time may be reversed through the use of cdrVMAT. More patients could be treated and the faster delivery times would also directly result in less patient-specific quality assurance time. As the delivery is only part of the entire treatment process other factors such as set-up may also play an important role. However, the fact that a reduced number of beams need to be checked during the setup would give advantage to the cdrVMAT technique presented. Both this study and Palma et al.Reference Palma, Vollans and James12 compared plans to five-field IMRT. If more fields were used the plan quality may have improved although couch attenuation and prolonged delivery because of more beams may have resulted in more complex analysis. Therefore, the five-field class solution was used in this study, which is routinely used at our centre and is similar to that used at other institutes.Reference Mott, Livsey and Logue25
As with previous studiesReference Alvarez-Moret, Pohl, Koelbl and Dobler32 the planning time was longer for the VMAT plans. Therefore, the improved delivery efficiency may be off-set with the slower planning times for cdrVMAT.
Dosimetric accuracy of the cdrVMAT plans was found to be 99·2 ± 1·1% using a 2D ionisation chamber device. This is similar to results presented with plans delivered to the same device using Rapidarc® plans, which had 98·7 ± 1·0% pixels passing 3%3 mm.Reference Lang, Reggiori and Puxeu Vaqué35 This also appears to be consistent with Feygelman et al.Reference Feygelman, Zhang and Stevens13 although no post planning editing of the MU is required in the solution presented in this paper. One of the reasons for accurate delivery was the avoidance of the couch during planning and delivery. The results presented also agree with those presented using the same planning system where the couch (although different type) was taken into accountReference Alvarez-Moret, Pohl, Koelbl and Dobler32 during delivery to the same ionisation chamber array. The ten clinically acceptable cdrVMAT plans were also delivered to an ArcCheck device and had 98·6 ± 0·4% of pixels passing 3%3 mm which was similar to the reported Rapidarc® plans having 98·0 ± 1·1% and 99·4 ± 1·1% pixels passing 3%3 mm for two different centres using the same device.
It has been postulated that given the limited availability of linacs with reliable variable dose rate capability, proof of equivalence between constant and vdrVMAT deliveries will allow wider adoption of VMAT delivery without additional linac resources.Reference Tang, Earl and Yu11 The work presented here shows that partial dual arc cdr plans are of similar quality to IMRT plans for treating radical prostate patients with the advantage of faster delivery times. The advantage over previously reported studiesReference Tang, Earl and Yu11 is the fact that these plans can be prospectively created using a commercially available treatment planning system removing the requirement for a recalculation using constant dose rate following optimisation using variable dose rates.
Conclusions
A method for Pareto front analysis is presented which specifies which plans are clinically acceptable. A dual arc VMAT is presented which does not require the couch to be modelled within the treatment planning system or the variable dose rate control system on the linear accelerator. The plans were shown to have accurate delivery with ∼45% faster delivery times compared with IMRT.
Acknowledgements
C.K.M. is supported by a Health & Social Care Research & Development Office of the Public Health Agency Training Fellowship Award. An evaluation VMAT system was supplied by Nucletron. The authors wish to acknowledge financial support from Cancer Research UK (Grant No. C1513/A7047 to KMP and C212/A11342 to ARH and SJM).