Introduction
Palliative radiotherapy aims to reduce symptoms, but not achieve cure, and results in pain relief in 60–80% of patients, Reference Chow, Zeng, Salvo, Dennis, Tsao and Lutz1 with one-third of those with bone metastases experiencing complete relief of pain. Reference Ejima, Ishihara, Miyawaki and Onal2 Single fields (one beam delivering the whole prescription) or parallel opposed pairs (two beams along the same axis) are widely used to deliver palliative radiotherapy, as they are quicker to position and plan, meaning patients spend less time on the treatment couch and there is less of a wait for treatment. The large volume of healthy tissue irradiated Reference Jones and Simone3,Reference Maranzano, De Angelis and Pergolizzi4 coupled with high doses per fraction (8 Gy per fraction or 20 Gy in 5 fractions) for bone metastases 5 radiotherapy-induced nausea and vomiting (RINV) is a common side effect of palliative radiotherapy to the upper abdomen, as it causes swelling of the epithelial and stromal tissue. Reference Maranzano, De Angelis and Pergolizzi4,Reference McKenzie, Zaki and Raman6–Reference Enblom, Bergius, Steineck, Hammar and Börjeson8 Patients may also experience side effects such as pain flare and others depending on the site; however, the focus of this study is on RINV. Nausea and vomiting can either occur in an acute phase within 24 hours of the treatment or in a delayed phase up to 10 days after radiotherapy, Reference Feyer, Maranzano, Molassiotis, Roila, Clark-Snow and Jordan9 this study was focussed on the acute phase. Evidence Reference i Garau, Calduch and López10 demonstrates 100% incidence of ‘very severe’ vomiting in patients receiving 6–8 Gy, with an onset of less than 30 minutes after exposure. Patients receiving 4–6 Gy also experienced a 100% incidence, yet the vomiting was ‘severe’ and the onset was under an hour. Some individual-level, patient-related risk factors for RINV have been suggested, such as patient age, sex, previous alcohol use and previous experiences of nausea and vomiting. Reference McKenzie, Zaki and Raman6 Nonetheless, evidence for this is limited and international guidelines Reference Feyer, Maranzano, Molassiotis, Roila, Clark-Snow and Jordan9 only cite concomitant chemotherapy as an individual risk factor. Research has shown that field sizes over 400 cm2 significantly increased the risk of RINV. Reference Maranzano, De Angelis and Pergolizzi4 Despite this, current guidelines/or local practice do not specify a field size threshold for which an anti-emetic is considered. Table 1 summarises the current tumour sites deemed to be at high or moderate risk.
Table 1. Emetogenic risk for treatment sites Reference Feyer, Maranzano, Molassiotis, Roila, Clark-Snow and Jordan9
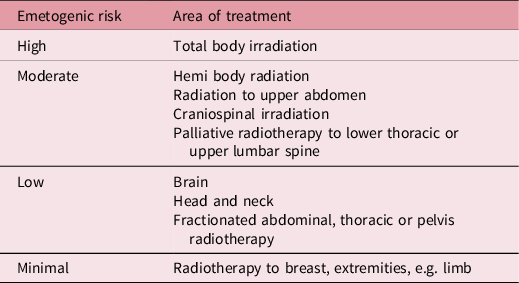
Single fraction (SF), palliative radiotherapy to some sites is particularly associated with a high or moderate risk of causing RINV, and it is common practice to prescribe these patients prophylactic antiemetic medication before treatment. Published evidence suggests, however, that protocols and practice vary considerably between clinical sites. Evidence indicates that between 50 and 80% of patients receiving radiotherapy suffer from RINV. Reference Feyer, Maranzano, Molassiotis, Roila, Clark-Snow and Jordan9 Despite this, antiemetics are only given to a small number of patients, with a 2010 study Reference Maranzano, De Angelis and Pergolizzi4 reporting that 17% of patients receiving radiotherapy were prescribed antiemetics and only 12·4% prophylactically. Due to the high number of patients experiencing RINV and the negative impact on the patient’s quality of life (QOL), Reference Yee, Drost, Zhang and Wan11 it is imperative that the importance of sufficient prophylactic treatment is recognised. Inadequately controlled nausea and vomiting can cause patients to delay or refuse subsequent treatment, therefore affecting treatment outcomes. Reference Feyer, Maranzano, Molassiotis, Roila, Clark-Snow and Jordan9 Additional impact on patient’s QOL Reference Pirri, Bayliss and Trotter12 arises from the resulting negative impact on social functioning and sleep and an increase in cancer distress.
Published guidelines Reference Roila, Molassiotis and Herrstedt13 can help staff decide whether a patient requires an antiemetic, with the literature recommending a range of 5-HT3 receptor antagonists as appropriate for the control of RINV. Reference Ettinger, Armstrong and Barbour14–Reference Ruhlmann, Jahn and Jordan16 Other antiemetics (outlined in Table 2) are also available and may be used by other centres. It has also been suggested that these patients could be offered proton pump inhibitors or H2 blocker therapy along with the patient eating smaller and more frequent meals instead of larger ones before treatment. Reference Koontz7 Treatments with a high emetogenic risk may also indicate concurrent use of dexamethasone to achieve control. Reference Ettinger, Armstrong and Barbour14 Unfortunately, research around RINV is limited and much less common than that on chemotherapy-induced nausea and vomiting (CINV). Despite the findings in this limited evidence, there is little clarity on which patients should receive medication. Research has shown under half of qualified consultants are aware of antiemetic guidance, Reference Dennis, Zhang and Lutz17 and therefore, the following audit was performed at a major clinical department in order to measure adherence to the prescription of prophylactic antiemetics protocol. The aim of the audit was to assess the extent to which prophylactic antiemetics are being prescribed to patients who are receiving SF radiotherapy to an area classed as a high or moderate emetogenic risk.
Table 2. Types of antiemetic that can be used for RINV Reference Ettinger, Armstrong and Barbour14,Reference Andrews and Sanger18,19

Method
A retrospective audit was carried out at a large regional cancer centre; patients included in the audit satisfied the inclusion criteria, derived from European Society for Medical Oncology (ESMO) guidelines Reference Roila, Molassiotis and Herrstedt13 as shown in Table 1. The audit was approved by the Trust’s ‘Quality Insurance and Clinical Audit Committee’. Data sets satisfying the inclusion criteria seen in Table 3 were initially located using a script to interrogate the scheduling software. Electronic notes for these patients were searched to extract data, and paper notes were searched if data was omitted from electronic versions. Paper prescription charts were examined to identify whether antiemetics had been prescribed or not; local policy for the acute phase indicated the prescription (carried out by a doctor or specialist nurse or radiographer) should be 8 mg Ondansetron once orally 30 minutes before treatment. This dose could be increased to twice daily if required, Reference Andrews and Sanger18,19 and the medication could be dispensed by radiographers, but not under patient group direction. All data were anonymised and collection was performed by two members of the research team. Data were stored in line with local governance and information technology policies and only accessible by the researchers. Descriptive statistics were used to summarise the data for analysis.
Table 3. Inclusion criteria and data extracted

Results
A total of 71 patients met the inclusion criteria. Of these, 11 sets of paper notes were unavailable so these patients were excluded, leaving a total of 60 patients who were included in the final data. It was found that of these patients, 50 were consented for the risk of nausea and/or vomiting (83·3%). On two of the consent forms (3·3%), the side effects noted were illegible, meaning that at least eight patients (13·3%) were not consented for RINV by their clinician. Consultant oncologists were the member of staff most likely to perform the consent of high and moderate RINV risk patients as seen in Table 3. Over 80% of patients consented had RINV included in the consent discussion, according to annotations; a breakdown of this by consenter role is shown in Table 4.
Table 4. RINV consent inclusion

* Key: AHP = specialist (radiographer or nurse) or metastatic spinal cord compression coordinator.
When prescription data were analysed, prophylactic antiemetics were only prescribed to 28 (46·7%) of all audited patients. Out of the 50 patients who provided informed consent including RINV side effects, only 24 (48%) were prescribed an antiemetic prior to their treatment. Of the 32 patients who were not prescribed Ondansetron, 10 were already on dexamethasone, 3 were inpatients and 1 patient was prescribed metoclopramide.
Other interesting findings included one patient being given an antiemetic by their general practitioner the day after treatment, with no record of it being prescribed at the radiotherapy department. Another patient who had not received prophylactic antiemetics was prescribed Ondansetron after treatment after complaining of nausea. One patient also experienced a single episode of vomiting, post treatment, after not being prescribed Ondansetron.
An analysis of the 6-week post treatment survival rate revealed that 22 patients died before 6 weeks; these patients may have not gained full benefit from their radiotherapy, as the side effects of palliative radiotherapy can take 6 weeks to resolve. Reference Spencer, Parrish, Barton and Henry20
Discussion
This audit aimed to determine if antiemetic prescribing was happening as per local guidance in accordance with published evidence. The data suggest that prophylactic prescription of antiemetics was only provided to 47% of patients, at high or moderate emetogenic risk. RINV was apparently not discussed during consent for 10 patients and, of the remaining 50, only 48% actually received an antiemetic. It would have been interesting to gather data concerning incidence of side effects for this cohort, but the detrimental impact on QOL can be inferred from the evidence base which cites nausea, vomiting, appetite loss, sleep disturbance, cancer distress, neuroticism and reduced social functioning Reference Pirri, Bayliss and Trotter12 as probable outcomes.
The side effects of palliative radiotherapy can last up to 6 weeks, 19 and the results show 20% of patients in this cohort died within 6 weeks of treatment. If these patients were not prescribed the appropriate antiemetic regime, their last few weeks of life may have been with a further reduced QOL; Reference Yee, Drost, Zhang and Wan11 however, it is difficult to ascertain if this was the case, as many of the patients died before the audit took place, and it was not deemed suitable to contact this group of patients in order to question them. Evidence-based prescription protocols could help to prevent this using a simple categorisation based on vertebral levels and field size.
A key objective of the audit was to determine if the correct patients are being consented for nausea and vomiting. According to the data, 83% of patients with a high or moderate emetogenic risk were consented for nausea and/or vomiting. For the remaining 17%, there are issues associated with the extent to which consent can be classed as ‘informed’. It is possible that some of these patients were already taking antiemetic medication; this was not identified within the audit. As dexamethasone itself reduces the chances of nausea and vomiting, Reference Pirri, Bayliss and Trotter12 it may be perceived that Ondansetron is not necessary for these patients. However, the evidence indicates that Ondansetron should always be given to high- and moderate-risk patients. Additional training and written information may help to refresh consenting staff on which treatment sites carry high or moderate RINV risk. Other antiemetics, such as metoclopramide, may have been given instead of Ondansetron as congenital long QT syndrome is a contraindication for Ondansetron. 21
In addition to the previously mentioned issue regarding existing medication, there is another noteworthy limitation to the audit that could affect the outcomes. An absence of prescription data in paper notes or annotation of electronic notes was interpreted as failure to prescribe. It may be that filing or annotation issues were responsible for some of the findings. Data concerning potential contraindications to medication were also not gathered. A final and minor limitation arose as a result of an illegible annotation. While electronic notes are helping to reduce this problem, it is clear that poor handwriting can impact on data collection.
Updating of existing local guidance is currently underway, guided by the results of this audit. Future iterations of this audit should hopefully provide assurance that improvements have been implemented.
Conclusion
The results of the audit show that antiemetic prescription guidance is currently not being followed rigorously and although there are limitations to this study, it suggests that improvements could be made to the service. Guidance is currently being updated, and future iterations of this audit will determine if this has led to improvements in the percentage of patients being prescribed prophylactic antiemetics. The results indicate that there may be value in developing a national protocol alongside further education and training concerning RINV and the effect of inadequate emesis control on the patient.
Acknowledgements
None.







