1. Introduction
Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2)—the cause of coronavirus disease 2019 (COVID-19)—first emerged in December 2019 in Wuhan, China [Reference Davies, McDougall and Yoong1–Reference Oliaei, SeyedAlinaghi and Mehrtak4]. The World Health Organization (WHO) declared a global COVID-19 pandemic in March 2020 [Reference Mehraeen, Salehi, Behnezhad, Moghaddam and Seyed5]. As of August 8th, 2022, COVID-19 has infected more than 581 million people and caused more than 6.4 million deaths worldwide [6]. SARS-Cov-2 is mainly an aerosol-born disease and infects new cases through respiratory droplet inhalation [7–Reference Seyed Alinaghi, Karimi and Barzegary9]. COVID-19 presents mostly with flu-like symptoms such as fever, chills, myalgia, dry cough, fatigue, back pain, headache, anorexia, diarrhea, anosmia (loss of smell sensation), and ageusia (loss of taste sensation) [10, Reference Byambasuren, Cardona, Bell, Clark, McLaws and Glasziou11]. However, 4–41% of total cases can be asymptomatic [10, Reference Byambasuren, Cardona, Bell, Clark, McLaws and Glasziou11]. Severe COVID-19 can cause acute respiratory distress syndrome (ARDS), which manifests with hypoxia, dyspnea, chest pain, altered consciousness, cyanosis, and eventual death. It mainly occurs among older adults (i.e., >65 years) and vulnerable populations including patients with chronic kidney, liver, or lung disease, diabetes mellitus, obesity, HIV infection, and smoking history [Reference Ramadori12].
Smokers are more predisposed to viral and bacterial pulmonary infections including influenza, tuberculosis, and bacterial pneumonia [Reference Atto, Eapen and Sharma13–Reference Tuder and Yun16]. In the first months of the pandemic in 2020, it was reported that Chinese patients with severe COVID-19 were mostly COPD patients or current smokers [Reference Guan, Ni and Hu17]. In addition, one of the earliest systematic reviews concluded that smoking is negatively associated with COVID-19 progression and prognosis [Reference Vardavas and Nikitara18]. Some studies have reported higher expression of angiotensin-converting enzyme 2 (ACE-2), which is the main receptor of SARS-CoV-2, in the lower respiratory airways of current smokers and COPD patients compared to nonsmokers, and stated that smoking and COPD could contribute to a higher COVID-19 incidence and relatively poorer outcomes [Reference Jacobs, Van Eeckhoutte and Wijnant19, Reference Leung, Yang and Tam20]. Conversely, some studies have reported lower levels of ACE-2 among smokers compared to nonsmokers [Reference Oakes, Fuchs, Gardner, Lazartigues and Yue21, Reference Wan, Shang, Graham, Baric and Li22], and one preliminary meta-analysis of five studies in China stated smoking may not be significantly associated with an increased risk of severe disease among COVID-19 patients [Reference Lippi and Henry23].
There have been five other meta-analyses conducted where the findings support the hypothesis that COPD and current smoking status contribute to worse progression and poor outcomes among COVID-19 patients [Reference Zhao, Meng and Kumar24–Reference Reddy, Charles, Sklavounos, Dutt, Seed and Khajuria28]. One of the meta-analyses specifically stated that smoking can have negative adverse impacts on disease severity and mortality among hospitalized COVID-19 patients, with more impact on nondiabetic younger patients [Reference Karanasos, Aznaouridis and Latsios27]. In addition, another meta-analysis specified that both current smoking and previous history of smoking increase COVID-19 severity significantly, while previous history of smoking increases the mortality risk [Reference Reddy, Charles, Sklavounos, Dutt, Seed and Khajuria28]. Now that almost three years has passed since the first report of SARS-CoV-2 in human population, and large sample-sized studies and more reliable data are available, further investigation of COVID-19 and smoking is warranted. The aim of this review was to examine the associations between COVID-19 and smoking status (current smoker or history of smoking), answer the controversial paradoxes, and fill the gaps in the literature.
2. Methods
The objective of this umbrella study was to explore the prevailing systematic review literature pertaining the associations between COVID-19 and smoking status (being a current smoker or having history of smoking) and effects of smoking on COVID-19 management and mortality. In order to substantiate the results, the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist was followed. Quality of studies was evaluated with the NIH quality assessment tool.
2.1. Data Sources
An extensive search of four online databases was performed which included PubMed, Scopus, Embase, and Web of Science as data sources. Articles were restricted to the English language, and the search was conducted up to July 27, 2022. The following keywords and their combinations were used during the search:
-
(a) “COVID-19” OR “Novel coronavirus” OR “2019-nCoV” OR “SARS-CoV-2” OR “SARS- CoV2” [Title/Abstract]
-
(b) “Smoking” [Title/Abstract]
-
(c) “Systematic review” [Title/Abstract]
-
(d) [A] AND [B] AND [C]
2.2. Study Selection
In order to improve the study selection process, a two-step method was employed. The first step consisted of screening literature with regard to titles and abstracts. This was done by five researchers. The second step was performed by another five researchers, involving screening of full texts that were potentially eligible. Articles that met the inclusion criteria were advanced to the next step of data extraction. Articles were included if they had a systematic review nature and were peer-reviewed report on smoking and COVID-19. The exclusion criteria included studies lacking published data investigations, nonhuman research studies, duplications, abstracts with deficient full texts, editorial letters, conference abstracts, case series, and case reports.
2.3. Data Extraction
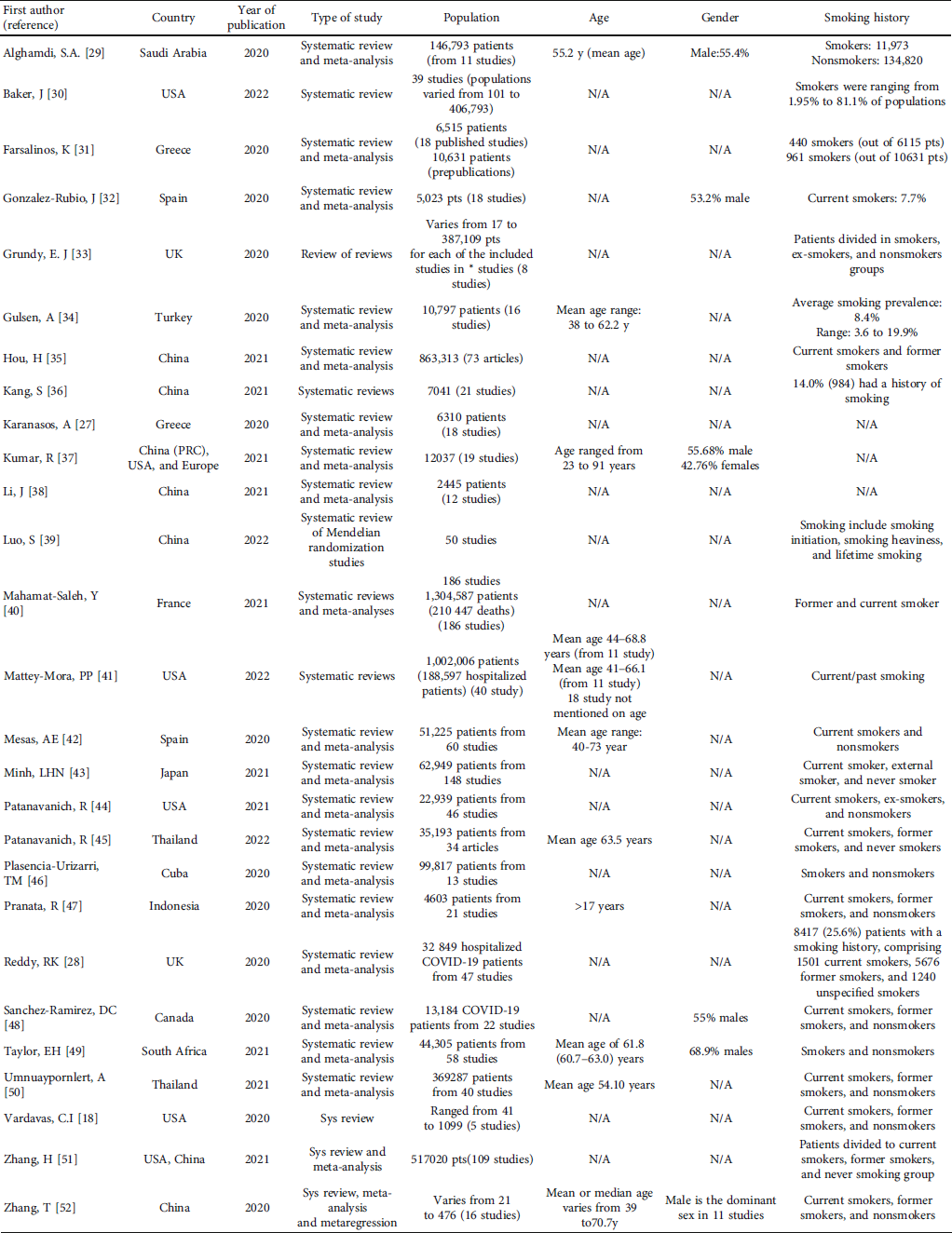
Data of publications having met the eligibility criteria and passing the second step of selection process was meticulously extracted and gathered in Table 1. Five researchers investigated the full texts and extracted these study requisites. Any duplicates were removed, and the accuracy of the extracted data was checked.
Table 1: Characteristics of studied items in the included papers.
2.4. Quality and Risk of Bias Assessment
Study quality and risk of bias was assessed with the National Institute of Health (NIH) Quality Assessment (QA) Tools for Case Series Studies. Two independent reviewers rated the quality of the included studies. Table 2 shows the results of the study quality and risk of bias. The scoring strategy of this tool has been explained at the bottom of this table.
Table 2: Quality ratings of included studies based on NIH quality assessment (QA) tool for case series studies.

Note: NIH: National Institutes of Health; CD: cannot determine; NR: not reported; NA: not applicable. *The NIH quality assessment tool for case series studies (https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools) contains nine questions: 1 = Was the study question or objective clearly stated?, 2 = Was the study population clearly and fully described, including a case definition?, 3 = Were the cases consecutive?, 4 = Were the subjects comparable?, 5 = Was the intervention clearly described?, 6 = Were the outcome measures clearly defined, valid, reliable, and implemented consistently across all study participants?, 7 = Was the length of follow-up adequate?, 8 = Were the statistical methods well-described?, 9 = Were the results well-described? (source: National Heart, Lung, and Blood Institute; National Institutes of Health; U.S. Department of Health and Human Services) https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools.
3. Results
The database search yielded 113 potential studies (after duplicates removed), and following the screening, a total of 27 articles met the eligibility criteria (Figure 1). The included systematic reviews were published between 2020 and 2022. One study included was designed as reviews of reviews [Reference Grundy, Suddek, Filippidis, Majeed and Coronini-Cronberg33].

Figure 1: PRISMA 2020 flow diagram of study retrieval process.
Included studies were from China, the USA, Spain, Greece, Thailand, Canada, Indonesia, Japan, and other countries. China (7 studies) and the USA (5 studies) were the countries most represented. The 27 systematic reviews included a wide range of studies (i.e., 8 to 186 studies) with different populations (from 17 to 1,304,587 patients among mentioned populations). Males were the dominant population in 6 studies [Reference Alghamdi, Alahmari and Bajari29, Reference González-Rubio, Navarro-López and López-Nájera32, Reference Kumar, Rai and Phukan37, Reference Sanchez-Ramirez and Mackey48, Reference Taylor, Marson and Elhadi49]. Table 1 shows the characteristics of the studies.
Smoking history was categorized as smokers and nonsmokers in some studies [Reference Plasencia-Urizarri, Aguilera-Rodríguez and Almaguer-Mederos46, Reference Taylor, Marson and Elhadi49], while most studies evaluated the smoking status as current and former smokers with comparisons between these groups [Reference Vardavas and Nikitara18, Reference Mahamat-Saleh, Fiolet and Rebeaud40–Reference Mesas, Cavero-Redondo and Álvarez-Bueno42, Reference Pranata, Soeroto and Huang47]. One study represented smokers based on smoking heaviness, lifetime smoking, and smoking initiation [Reference Luo, Liang, Wong, Schooling and Au Yeung39].
Most of the studies concluded that smoker’s with COVID-19 had a worse outcome and higher mortality rate [Reference Karanasos, Aznaouridis and Latsios27, Reference Baker, Krishnan, Abroms and Berg30, Reference Gülsen, Yigitbas, Uslu, Drömann and Kilinc34, Reference Kang, Gong and Yuan36]. Evaluating the hospital admission rate, most of the studies showed increased risk of hospital admissions in smokers [Reference Vardavas and Nikitara18, Reference Alghamdi, Alahmari and Bajari29, Reference Baker, Krishnan, Abroms and Berg30], while one study showed that smokers had lower risk of hospital admission [Reference Grundy, Suddek, Filippidis, Majeed and Coronini-Cronberg33].
The majority of studies found that smoking was associated with increased risk for mechanical ventilation [Reference Vardavas and Nikitara18, Reference Alghamdi, Alahmari and Bajari29, Reference Baker, Krishnan, Abroms and Berg30]; however, there was one study showing no association between smoking status and risk of mechanical ventilation [Reference Zhang, Ma and Han51]. These results are shown in Table 3.
Table 3: Description of the findings reported in the eligible studies.

4. Discussions
According to the NIH’s LitCovid database, more than 291,000 articles related to COVID-19 have been published to date, which shows the explosion of research in this field. This incredible amount of research has increased the information on different aspects of the disease. However, knowledge is still lacking in some areas with further research required to better understand COVID-19 and its effects. This umbrella review is aimed at organizing and updating the existing body of literature on the effects of smoking on COVID-19.
The overall findings support the hypothesis of increased severity of disease in COVID-19 smoker patients. Specifically, smoking is linked to more advanced COVID-19 outcomes, as manifested by the necessity for ICU admission, mechanical ventilation, and COVID-19-related death. Nevertheless, only a few studies demonstrated no significant link between smoking and COVID-19-related mortality [Reference Baker, Krishnan, Abroms and Berg30] and mechanical ventilation [Reference Zhang, Ma and Han51]. Additionally, only one study found no link between smoking and an increased risk of death from COVID-19 [Reference Karanasos, Aznaouridis and Latsios27]. Notably, a study in England, that investigated approximately 17 million patient documents, discovered that increased COVID-19-related mortality linked to smoking no longer remained significant after adjustment for the presence of preexisting chronic pulmonary disease. This suggests that smoking-induced comorbidities may be the cause of the overall death toll among COVID-19 smoker patients [Reference Williamson, Walker and Bhaskaran53]. The results emphasize the need for further research elucidating the mechanisms by which smoking increases the incidence of unfavorable outcomes in COVID-19 patients.
Various potentially harmful compounds are present in tobacco products. Moreover, further chemicals are formed during aerosolization as a result of combustion or heating. Previous studies have demonstrated that pulmonary epithelium and vascular endothelium are both damaged by harmful compounds in cigarette smoke. The mucociliary clearance and epithelial barrier are compromised by damage to epithelial cells. Additionally, injured cells release modified molecules that activate specific lung receptors, activating acquired and innate immune responses [Reference Nyunoya, Mebratu, Contreras, Delgado, Chand and Tesfaigzi54]. Through a variety of mechanisms including the direct effect of nicotine, reperfusion injury after carbon monoxide-induced hypoxia, and particulate matter’s abundance, tobacco smoke ingredients cause oxidative stress [Reference Qasim, Alarabi, Alzoubi, Karim, Alshbool and Khasawneh55, Reference Piantadosi56].
The angiotensin II conversion enzyme-2 (ACE2) receptor, which is abundant in mucosal respiratory epithelial cells, has been associated with COVID-19 infection. It is probably the most reasonable explanation for the potential increased risk of death among smokers. Infection by the host-virus binding to the ACE2 receptors is likely a critical stage in SARS-CoV-2 infection [Reference Arcavi and Benowitz57–Reference Strzelak, Ratajczak, Adamiec and Feleszko59]. Smokers have significantly higher levels of pulmonary ACE2 gene expression compared to nonsmokers [Reference Cai, Bossé, Xiao, Kheradmand and Amos60]. Tobacco use can cause oxidative stress and inflammation in the lungs, making smokers more susceptible to bacterial or viral diseases [Reference Bauer, Morissette and Stämpfli58, Reference Yao and Rahman61]. Oxidative stress reduces epithelial permeability, which may have substantial consequences for smokers with COVID-19 disease [Reference Wiener, Cao, Hinds, Ramirez and Williams62, Reference Meng, Li and Zhou63]. Likewise, smoking leads to cardiovascular disease, chronic lung illness, diabetes, and other comorbidities that are related to worse outcomes in patients with COVID-19 infection [Reference Richardson, Hirsch and Narasimhan64].
On the contrary, there is evidence demonstrating that smoking may exert positive effects on COVID-19 disease severity, mediated by nicotine [Reference Zhang, Ma and Han51]. It is important to keep in mind that specific cigarette ingredients, like nicotine, may affect ACE2 differently from entire cigarettes [Reference Ferrari, Raizada and Fior-Chadi65]. The underlying mechanism might be attributed to the evidence showing nicotine might decrease tumor necrosis factor (TNF) expression in airway epithelial cells [Reference Li, Zhou, Kolosov and Perelman66]. Moreover, nicotine may act as an agonist of the cholinergic anti-inflammatory pathway, which regulates the immune response and inflammatory reaction [Reference Wang, Yu and Ochani67, Reference Tracey68].
Factors responsible for higher susceptibility of smokers to COVID-19 are briefly discussed here to better understand the mechanisms about how smoking can affect the human body:
-
(i) Smoking causes significant pathological changes including the mucosal epithelial barrier and an increase in the permeability of epithelial cells, which makes smokers more prone to be defeated against the virus invasion [Reference Aghapour, Raee, Moghaddam, Hiemstra and Heijink69]
-
(ii) Angiotensin-converting enzyme 2 (ACE 2) is defined as the principal receptor for SARS-CoV-2 virus to enter the host cell. ACE 2 expression is upregulated in the small airway epithelium of smokers, so the virus tends to invade host cells more easily. This upregulated receptor expression is one of the negative influences of oxidative stress due to smoking [Reference Leung, Yang and Tam20]
-
(iii) Smoking can reduce the function of the immune system by causing a reduction in the number of CD4 + T cell (also named T helper cell, which activates macrophage or B cell), inhibiting the production of interleukin-22 (which moderates lung inflammation) and also, by promoting the secretion of catecholamines (which will weaken the immune system) [Reference Nguyen, Torres, Agrawal and Agrawal70, Reference Yue, Jian, Xiaoqian and Qiang71]
-
(iv) The most serious complication of COVID-19 disease is acute respiratory distress syndrome (ARDS), as a result of the cytokine storm. In this situation, large amounts of proinflammatory cytokines and chemokines such as IP-10, IL-6, TNF-α, IFN-γ, IL-2, IL-7, and GM-CSF are released and entail severe immune system response which eventually causes lung inflammation and damage. In former smokers, the expression of IL-6, TNF-α, and other proinflammatory factors are increased [Reference Li, Geng, Peng, Meng and Lu72]
Although most of the reviewed articles support the hypothesis that nicotine, the main component of cigarettes, increases the odds of developing severe illness of COVID-19, it is unknown whether the harm is related to nicotine or other toxic ingredients in cigarettes. Even some articles indicate that nicotine may have anti-inflammatory effects. Nicotine has been found to prevent acute lung damage and restrain the expression of tumor necrosis factor (TNF), which plays role in the inflammatory response [Reference Li, Zhou, Kolosov and Perelman66]. Furthermore, nicotine is an agonist of the cholinergic anti-inflammatory pathway that modulates immune and inflammatory reaction [Reference Zhang, Ma and Han51, Reference Tracey73].
There have been a few original research studies comparing former and current smokers. The lung may heal when someone quits smoking, which may bias the findings if former smokers are part of the exposed group since one study found that the death rate among former smokers reduces with age [Reference Patanavanich and Glantz44, Reference Patanavanich, Siripoon, Amponnavarat and Glantz45]. As a result, the frequency of smokers may have been underestimated, and some former smokers may have been incorrectly classified as nonsmokers [Reference Zhang, Ma and Han51]. As a result, only a few systematic reviews compared the features of these two groups. Three studies found that current smokers had lower risks of an adverse outcome than former smokers [Reference Farsalinos, Barbouni, Poulas, Polosa, Caponnetto and Niaura31, Reference Hou, Li and Zhang35, Reference Zhang, Ma and Han51]. This might be because former smokers are more likely to be older, smoked for a longer period than current smokers, or because they have concomitant conditions such as asthma or COPD as a result of smoking [Reference Zhang, Ma and Han51]. Another factor might be that current smokers reported their status less than former smokers. [Reference Umnuaypornlert, Kanchanasurakit, Lucero-Prisno and Saokaew50], although a study found that current smokers had a greater likelihood of adverse outcomes than former smokers and nonsmokers [Reference Pranata, Soeroto and Huang47].
Overall, compared with nonsmokers, smokers have higher risks of severe forms of the disease and hospitalization, and mortality [Reference Vardavas and Nikitara18, Reference Luo, Liang, Wong, Schooling and Au Yeung39, Reference Clift, Von Ende and San Tan74]. On the contrary, the severity of COVID-19 was not associated with current or former smoking but with the comorbidities caused by smoking [Reference Matsushita, Yokoyama and Hayakawa75, Reference Korzeniowska, Ręka, Bilska and Piecewicz-Szczęsna76]. In this study, the incidence of infection by SARS CoV-2 virus in smokers and nonsmokers cannot be evaluated clearly due to the lack of data at this present time.
5. Conclusion
There is strong evidence that smoking increases the risks of disease severity/progression, hospitalization, and mortality among COVID-19 patients. Encouraging smokers to quit using, early initiation of treatment after the onset of symptoms, timely vaccination, and promoting other preventive behaviors by public health providers can control the possibility of these people getting the infection and a better prognosis. Vaccination of smokers should be done completely among the priority groups.
Data Availability
The authors stated that all information provided in this article could be shared.
Additional Points
Implications. (i) There is strong evidence that smoking increases the risks of disease severity/progression, hospitalization, and mortality among COVID-19 patients. (ii) Encouraging smokers to quit using, early initiation of treatment after the onset of symptoms, timely vaccination, and promoting other preventive behaviors by public health providers can control the possibility of these people getting the infection and a better prognosis. (iii) Vaccination of smokers should be done completely among the priority groups.
Disclosure
The present study was conducted in collaboration with the Khalkhal University of Medical Sciences, Iranian Institute for Reduction of High-Risk Behaviors, Tehran University of Medical Sciences, and the University of Sydney.
Conflicts of Interest
The authors declare that there is no conflict of interest regarding the publication of this manuscript.
Authors’ Contributions
Esmaeil Mehraeen and SeyedAhmad SeyedAlinaghi are responsible for the conception and design of the study; Amir Masoud Afsahi and Ramin Shahidi for the acquisition of data; Esmaeil Mehraeen, Shaghayegh Kianzad, Zahra Pashaei, Maryam Mirahmad, Pooria Asili, Hengameh Mojdeganlou, Armin Razi, Paniz Mojdeganlou, Iman Amiri Fard, Sara Mahdiabadi, Arian Afzalian, Mohsen Dashti, Afsaneh Ghasemzadeh, Zohal Parmoon, and Hajar Badri for the drafting of the article; SeyedAhmad SeyedAlinaghi, Daniel Hackett, and Amir Masoud Afsahi for revising it critically for important intellectual content; and SeyedAhmad SeyedAlinaghi, Esmaeil Mehraeen, and Daniel Hackett for the final approval of the version to be submitted.
Supplementary Materials
Supplementary table 1: description of the findings reported in the eligible studies. (Supplementary Materials)






