INTRODUCTION
Children with epilepsy are at greater risk for psychopathology when compared with children in the general population (Davies, Heyman, & Goodman, Reference Davies, Heyman and Goodman2003; Rutter, Graham, & Yule, Reference Rutter, Graham and Yule1970) and children with other neurological and chronic medical conditions (Rodenburg, Stams, Meijer, Aldenkamp, & Deković, Reference Rodenburg, Stams, Meijer, Aldenkamp and Deković2005). Depression is a common clinical feature observed in pediatric epilepsy, with prevalence estimates ranging from 20 to 40% (Ekinci, Titus, Rodopman, Berkem, & Trevathan, Reference Ekinci, Titus, Rodopman, Berkem and Trevathan2009). Among children and adolescents with epilepsy, patients with temporal lobe epilepsy (TLE), the most common form of focal epilepsy, have higher rates of depression features (Salpekar et al., Reference Salpekar, Berl, Havens, Cushner‐Weinstein, Conry, Pearl and Gaillard2013; Schraegle & Titus, Reference Schraegle and Titus2017a; Titus, Kanive, Sanders, & Blackburn, Reference Titus, Kanive, Sanders and Blackburn2008), and recent research by Schraegle and Titus (Reference Schraegle and Titus2017a) demonstrated that children with TLE incurred over a two-fold risk for depression features. Because it is often under-recognized and under-treated in pediatric TLE (Kavanaugh, Scarborough, & Salorio, Reference Kavanaugh, Scarborough and Salorio2015), depression in this population can contribute to psychosocial dysfunction and poorer health-related quality of life (HRQOL) (Plioplys, Reference Plioplys2003; Schraegle & Titus, Reference Schraegle and Titus2017a).
The underlying mechanisms that contribute to depression in pediatric TLE are not well understood, but they are thought to involve multiple factors, spanning neurocognitive, sociodemographic, and seizure-specific influences. It has been posited that TLE and depression in adults share a similar neuropathogenic process (Gilliam et al., Reference Gilliam, Maton, Martin, Sawrie, Faught, Hugg and Kuzniecky2007; Richardson et al., Reference Richardson, Griffith, Martin, Paige, Stewart, Jones and Seidenberg2007) with imaging investigations describing similar disruptions to the hippocampus, amygdala, and long-range frontal lobe projections (Keller, Baker, Downess, & Roberts, Reference Keller, Baker, Downes and Roberts2009; Sheline, Reference Sheline2003). Not surprisingly, hippocampal abnormalities, such as hippocampal sclerosis (HS) (i.e., severe gliosis and cell loss in the hippocampus), in both adult TLE and depression are key contributors to frontotemporal network dysfunction (Focke et al., Reference Focke, Yogarajah, Bonelli, Bartlett, Symms and Duncan2008; Kaiser, Andrews-Hanna, Wager, & Pizzagalli, Reference Kaiser, Andrews-Hanna, Wager and Pizzagalli2015). However, despite these overlapping neural substrates, the presence of cognitive risk factors for depression in pediatric epilepsy has received only limited attention to date.
Within the broader population of pediatric depression, deficits in executive function (EF) are frequently reported. For example, youth with major depressive disorder (MDD) demonstrate deficits in phonemic fluency (Cataldo, Nobile, Lorusso, Battaglia, & Molteni, Reference Cataldo, Nobile, Lorusso, Battaglia and Molteni2005; Klimkeit, Tonge, Bradshaw, Melvin, & Gould, Reference Klimkeit, Tonge, Bradshaw, Melvin and Gould2011), set-shifting/cognitive flexibility (Holler, Kavanaugh, & Cook, Reference Holler, Kavanaugh and Cook2014), and auditory attention and working memory (Klimkeit et al., Reference Klimkeit, Tonge, Bradshaw, Melvin and Gould2011). A recent meta-analysis by Wagner, Müller, Helmreich, Huss, and Tadić (Reference Wagner, Müller, Helmreich, Huss and Tadić2015) highlighted deficits associated with pediatric MDD in immediate and delayed verbal recall, sustained attention, inhibitory control, semantic fluency, and planning ability. Importantly, impaired behavioral regulation has been identified as a central feature of both adult MDD (Gyurak, Goodkind, Kramer, Miller, & Levenson, Reference Gyurak, Goodkind, Kramer, Miller and Levenson2012; Ladouceur et al., Reference Ladouceur, Dahl, Williamson, Birmaher, Ryan and Casey2005) and TLE (Espinosa et al., Reference Espinosa, Machado, González, González, Montoto and Sotomayor2010).
Adults with TLE have been found to have EF deficits in cognitive flexibility, abstraction, set-shifting, and problem solving (Cocoran & Upton, Reference Corcoran and Upton1993; Hermann, Seidenberg, & Bell, Reference Hermann, Seidenberg and Bell2002; Igarashi et al., Reference Igarashi, Oguni, Osawa, Awaya, Kato, Mimura and Kashima2002), and emerging research in pediatric TLE has demonstrated EF deficits in working memory (Gottlieb, Zelko, Kim, & Nordli, Reference Gottlieb, Zelko, Kim and Nordli2012), sustained attention (Lopes, Simoes, Robalo, Fineza, & Gonçalves, Reference Lopes, Simoes, Robalo, Fineza and Gonçalves2010; Rzezak et al., Reference Rzezak, Fuentes, Guimarães, Thome-Souza, Kuczynski, Li and Valente2007), and cognitive flexibility (Igarashi et al., Reference Igarashi, Oguni, Osawa, Awaya, Kato, Mimura and Kashima2002; Rzezak et al., Reference Rzezak, Fuentes, Guimarães, Thome-Souza, Kuczynski, Guerreiro and Valente2009). Rzezak et al. (Reference Rzezak, Fuentes, Guimarães, Thome-Souza, Kuczynski, Guerreiro and Valente2009) reported that 77% of youth with TLE had EF deficits on the Wisconsin Card Sorting Test (WCST). With exception to youth with frontal lobe epilepsy (FLE), youth with TLE exhibit a higher rate of EF impairment than youth with generalized epilepsy or focal epilepsy from other regions of the brain (Rzezak, Valente, & Duchowny, Reference Rzezak, Valente and Duchowny2014).
Corcoran and Upton (Reference Corcoran and Upton1993) postulated that HS in adult TLE is likely a key contributor to frontotemporal network dysfunction which is thought to underlie executive deficits. More recent neuroimaging studies in adult TLE have correlated executive performance to diminished left dorsal prefrontal cortex and left hippocampal volumes (Keller et al., Reference Keller, Baker, Downes and Roberts2009) and shown diminished left frontal activation on a verbal working memory task in patients with left HS (Campo et al., Reference Campo, Maestu, Garcia-Morales, Gil-Nagel, Strange, Morales and Ortix2009). While the relationship between executive dysfunction and HS has been identified in adults, this relationship has yet to be fully examined in the pediatric TLE literature.
Additional seizure-specific variables are also believed to contribute to the risk for depression in youth with epilepsy, but previous literature has been equivocal about the role of many of the most commonly reported clinical features. For example, the evidence linking seizure frequency, age at seizure onset, and number of anti-epileptic medications with depression is mixed (Austin et al., Reference Austin, Harezlak, Dunn, Huster, Rose and Ambrosius2001; Thome-Souza et al., Reference Thome-Souza, Kuczynski, Assumpção, Rzezak, Fuentes, Fiore and Valente2004), although longer duration of epilepsy may have a somewhat stronger association (Austin et al., Reference Austin, Harezlak, Dunn, Huster, Rose and Ambrosius2001; Oguz, Kurul, Dirik, & Eylül, Reference Oguz, Kurul, Dirik and Eylül2002). The nature of these relationships has not been well examined in the pediatric literature, and there is no information about whether risk factors remain consistent throughout development. Additionally, despite evidence in the adult literature of a relationship between epilepsy-related brain pathology and depression, the pediatric literature has not examined such associations. For example, Gilliam et al. (Reference Gilliam, Maton, Martin, Sawrie, Faught, Hugg and Kuzniecky2007) identified HS as a significant risk factor for depression in adults with TLE; however, no studies to date consider whether HS increases the risk for psychopathology in youth with TLE.
Sociodemographic risk factors have long been shown to be a risk-factor for depression in the general population, and the same is true in the context of epilepsy. For instance, previous research has shown that up to 50% of patients with epilepsy and depression have a family history of depression (Plioplys, Reference Plioplys2003). Thome-Souza et al. (Reference Thome-Souza, Kuczynski, Assumpção, Rzezak, Fuentes, Fiore and Valente2004) reported that a family history of psychopathology increases the risk of depression in younger children. Similarly, higher family stress has been found to correlate with increased behavior problems in youth, even when controlling for seizure variables (Ekinci et al., Reference Ekinci, Titus, Rodopman, Berkem and Trevathan2009).
Study Aims
Despite the known clinical relationship between pediatric TLE and depression, no studies to date have explored neurocognitive, sociodemographic and seizure-specific vulnerabilities in this population. Therefore, the current study examined the following research questions: (1) Are there cognitive risk factors for depression in pediatric epilepsy? (2) Do cognitive risk factors contribute uniquely to depression in pediatric epilepsy, or is it better explained by sociodemographic or seizure-specific factors? (3) Is HS associated with EF deficits in youth with TLE? (4) Does the presence of HS incur greater risk for depression in children and adolescents with TLE?
METHODS
Participants
Youth between the ages of 8 and 16 years with a prior diagnosis of TLE who were referred for epilepsy treatment at a tertiary care health system were eligible for inclusion. Children were referred for neuropsychological services by their treating neurologist or epileptologists based upon clinical need. Specifically, approximately 42% of the sample was referred as candidates for surgery to treat intractable seizures. The remainder of the sample was composed of children with TLE who were experiencing cognitive difficulties that would warrant a neuropsychological evaluation. TLE was confirmed by board-certified epileptologists via clinical evaluation, ictal electroencephalograph (EEG) monitoring, and concordant interictal EEG findings, consistent with International League Against Epilepsy criteria (Engel, Reference Engel2001). Seizure laterality was also investigated; however, those children with bilateral epileptiform activity (n=2) were excluded due to incomplete data. HS was identified by magnetic resonance imaging (MRI), namely increased signal intensity on T2-weighted and FLAIR images, and verified by a pediatric neuroradiologist.
Approval by the Seton Institutional Review Board and written consent by parents were obtained to access archival data. Exclusion criteria included the presence of extratemporal epileptiform activity on EEG, previous neurosurgery, diagnosis of hearing or visual impairment, or a positive history of psychosis. Children were also excluded if caregivers could not speak or read English.
Sociodemographic and Epilepsy-Specific Variables
Demographic and seizure-related variables were taken directly from a combination of prior medical records, parent interview, and a standardized family information questionnaire completed by a parent the day of evaluation. The following demographic data were collected: participant age, gender, ethnicity, maternal education level, parental marital/relationship status, and family stress (i.e., “Please rate the overall degree of you family’s stress in the past year”; coded: 1,=very little stress, 2=lower than typical, 3=typical stress, 4=higher than typical, and 5=very high stress).
Parental history of psychopathology was coded as 1 (positive identification) or 0 (no history identified). Parental psychiatric history included depressive disorders, anxiety disorders, and bipolar disorders. Seizure-related variables included the following: age at seizure onset, number of epilepsy-related medications (antiepileptic drugs [AEDs]), number of failed AEDs, seizure frequency (coded: 0=never, 1,=yearly, 2=quarterly, 3=monthly, 4=weekly, and 5=daily), duration of epilepsy (i.e., time since the first seizure), and positive identification of HS (coded: 0=negative and 1=positive identification).
Neuropsychological and Behavioral Measures
All children and adolescents were administered neuropsychological tests by individuals trained in standardized testing procedures. Neuropsychological variables used in the present study are outlined in Table 1. Additionally, caregiver ratings on the Behavior Assessment for Children-2 (BASC-2) are defined as follows: T-scores at or below 59 are considered in the “Normal” range; T-scores of 60–69 are considered “At-Risk” and indicate behaviors that may warrant clinical attention; and T-scores≥70 suggest “Clinically Significant” levels and represents a high level of maladjustment warranting clinical attention (Reynolds & Kamphaus, Reference Reynolds and Kamphaus2004).
Table 1 Overview of neuropsychological and behavioral measures
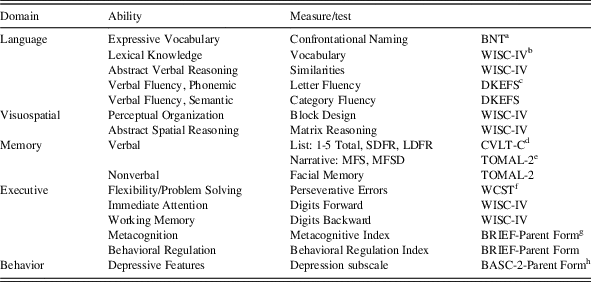
a Boston Naming Test (Kaplan, Goodglass, Weintraub, Reference Kaplan, Goodglass and Weintraub2001)
b Wechsler Intelligence Scale for Children-Fourth Edition (Wechsler, Reference Wechsler2003).
c Delis-Kaplan Executive Function System (Delis, Kaplan, Kramer, Reference Delis, Kaplan and Kramer2001).
d California Verbal Learning Test-Children’s Version (Delis, Kramer, Kaplan, Ober, Reference Delis, Kramer, Kaplan and Ober1994).
e Test of Memory and Learning, 2nd Edition (Reynolds & Voress, Reference Reynolds and Voress2007).
f WCST: Wisconsin Card Sorting Test (Heaton, Chelune, Talley, Kay, & Curtiss, Reference Heaton, Chelune, Talley, Kay and Curtiss1993).
g Behavior Rating Inventory of Executive Function: Parent Rating Scale (Gioia, Isquith, Guy, and Kenworthy, Reference Gioia, Isquith, Guy and Kenworthy2000).
h Behavior Assessment System for Children – 2: Parent Rating Scale (Reynolds & Kamphaus, Reference Reynolds and Kamphaus2004).
Statistical Analysis
To better characterize how sociodemographic sample features relate to depression, chi-square tests were conducted on demographic variable frequencies at three levels of depression severity (i.e., Normal, At-Risk, and Clinically Significant). Variables were dichotomized as follows: maternal education (less than high school vs. high school and beyond), parent relationship status (divorced/separated vs. married/together), parental psychiatric history (positive vs. negative), and family stress (“higher than typical stress” and “very high stress” vs.≤“typical stress” ratings). Depression severity was then examined within the following variables: maternal education less than high school, parents divorced or separated, positive parental psychiatric history, and family stress≥“higher than typical.”
All neuropsychological measures were converted to age-corrected standard scores using the best available norms. Correlations (i.e., Pearson, point biserial, as appropriate) were conducted to identify significant demographic and seizure-specific variables for statistical control as well as to characterize the associations between neuropsychological variables and the BASC-2 depression subscale. Next, a simultaneous linear regression was conducted to determine unique predictors of depression. Multicollinearity was tested and found not to be present as predictor inflation factor (variance inflation factor) and collinearity tolerance were within acceptable limits. Partial correlations were also examined to consider shared variance and the unique contribution of each variable to depression.
Three separate analyses of variance (ANOVAs) were conducted to test the main effect of HS. Mediation was assessed using both the traditional causal steps approach and non-parametric bootstrapping procedures (Preacher & Hayes, Reference Preacher and Hayes2004), and a binary logistic regression was conducted to determine whether HS served as a specific risk factor for depression.
Because of the exploratory nature of the study and the need to balance Type I and Type II error, the αlevel for all significance tests was set at 0.05 a priori. However, only moderate-to-large correlations (r≥| 0.32 |; p≤.01) were interpreted as evidence of significant associations between variables. All analyses were conducted using SPSS version 21.0 (SPSS Inc., Chicago IL).
RESULTS
Sample Characteristics
Table 2 lists sociodemographic and seizure-specific sample characteristics. The study included 62 children and adolescents with TLE (64% male) aged 8 to 16 years (M=12.62; SD=2.26). Children and adolescents came from various ethnic backgrounds, with 45% Caucasian, 26% African American, 19% Hispanic, and less than 10% identifying as Asian or Polynesian. Around 58% of the sample came from divorced or separated households, with the majority of mothers attending at least “some college.” Positive or ongoing psychiatric history in parents was identified in 58% and overall family stress averaged between “typical stress” to “higher than typical stress.”
Table 2 Descriptive values for sociodemographic and epilepsy variables
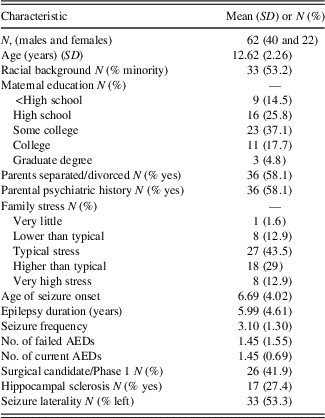
Most patients experienced seizure onset in childhood (M=6.69; SD=4.02), with roughly 16% of the sample reporting seizure onset in the first year of life. Seizure frequency occurred yearly in 15%, quarterly in approximately 10%, monthly in approximately 40%, weekly in approximately 19%, and daily seizures in 16%. Medication regimen for children and adolescents averaged one current AED (M=1.45; SD=0.69) and around one failed AED trial (M=1.45; SD=1.55). HS was identified in 17 of patients (27%), and 53% of youth in the sample had seizures lateralized to the left temporal lobe.
As seen in Table 3, neuropsychological performance predominantly fell within the low average range across domains. Parent rated depression features on the BASC-2 fell in the At-risk range as a group (M=60.55; SD=14.38), and 21 children (33.9%) were rated as having Clinically Significant features of depression. These rates are consistent with previous research (Salpekar et al., Reference Salpekar, Berl, Havens, Cushner‐Weinstein, Conry, Pearl and Gaillard2013; Schraegle & Titus, Reference Schraegle and Titus2017a; Titus et al., Reference Titus, Kanive, Sanders and Blackburn2008).
Table 3 Neuropsychological profile of the current sample
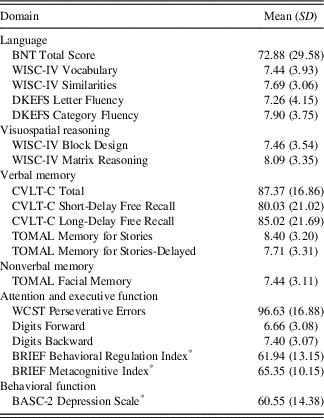
Note. Standard score, mean=100, SD=15; T-score, mean=50, SD=10; scaled score, mean=10, SD=3. BRIEF and BASC ratings use T-scores (*), and higher scores indicate greater levels of impairment.
BASC-2=Behavior Assessment System for Children – 2: Parent Rating Scale; BNT=Boston Naming Test; BRIEF=Behavior Rating Inventory of Executive Function: Parent Rating Scale; CVLT-C=California Verbal Learning Test-Children’s Version; DKEFS=Delis-Kaplan Executive Function System; TOMAL=Test of Memory and Learning, 2nd Edition; WCST=Wisconsin Card Sorting Test; WISC-IV=Wechsler Intelligence Scale for Children-Fourth Edition.
Sociodemographic Features by Depression Severity
The percentages of various sociodemographic sample features grouped by depression severity (i.e., Normal, At-Risk, and Clinically Significant) are displayed in Figure 1. Group differences on depression severity were not identified for maternal education, parent relationship status, or parental psychiatric history (p>.05). However, elevated levels of family stress were more prevalent in youth with clinically elevated levels of depression compared to those with at-risk ratings (χ2=5.22; p=.022) and ratings falling in the normal range (χ2=7.88; p=.005) (see Table 4).
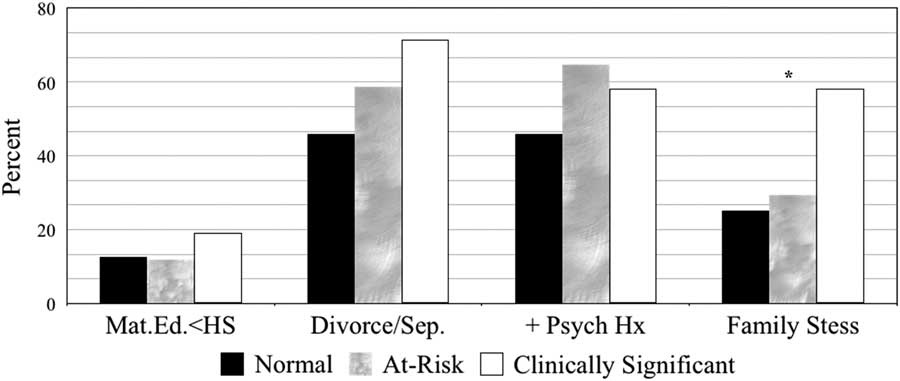
Fig. 1 Sociodemographic features by depression severity.
Note. Normal, T-scores≤59; at-risk, T-scores between 60-69; and clinically significant and T-scores≥70. Mat.Ed. <HS=Maternal education less than high school; Divorce/Sep.=Parents separated or divorced;+Psych Hx=Positive parental psychiatric history
Table 4 Counts and percentages of sociodemographic features by depression severity

*Significant difference between clinically elevated and normal; p<0.01. Significant difference between clinically elevated and at-risk; p<0.05.
Correlations Between Neuropsychological Variables and Depression Ratings
Pearson’s correlations between neuropsychological measures and parent BASC-2 depression ratings are presented in Table 5. The results indicated significant associations between depression ratings and perseverative errors on the WCST (r=–0.426; p<.001) and the BRIEF BRI (r=0.498; p≤.0001). Moderate associations were indicated between depression ratings and CVLT-C Total Score (r=–0.258; p=.043), CVLT-C Short-Delay Free Recall (r=–0.276; p=.030), WISC-IV Digits Forward (r=–0.270; p=.034), and metacognition on the BRIEF (MI; r=0.281; p=.027), but these associations did not survive correction for multiple comparisons.
Table 5 Bivariate correlations between neuropsychological variables and BASC-2 depression ratings
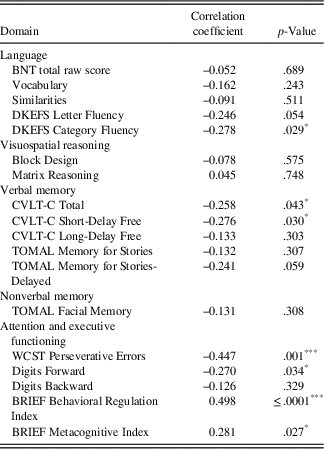
BRIEF=Behavior Rating Inventory of Executive Function: Parent Rating Scale; BNT=Boston Naming Test; CVLT-C=California Verbal Learning Test-Children’s Version; DKEFS=Delis-Kaplan Executive Function System; TOMAL=Test of Memory and Learning, 2nd Edition; WCST=Wisconsin Card Sorting Test.
* p<.05.
** p<.01.
*** p<.001.
When examining sociodemographic and seizure-specific variables, the sole significant correlation was a positive association between family stress and depression ratings (r=0.377; p=.003). There was a trend for HS to be related to depression ratings (r=0.316; p=.012), but maternal education (r=−0.163), parental psychiatric history (r=0.227), age of seizure onset (r=−0.07), epilepsy duration (r=0.091), seizure frequency (r=−0.047), number of failed AEDs (r=−0.071), and number of current AEDs (r=0.124) were unrelated to depression ratings (p>.05).
Defining Specific Predictors of Depression in Pediatric TLE
To determine whether EF variables contributed any unique variance to the prediction of youth depression ratings above and beyond family stress, a linear regression was conducted (Table 6). In an effort to maintain adequate power for the present analysis, predictors were chosen based on moderate-to-large associations with depression ratings (p≤.01). Partial and semipartial correlations were examined to explore the amount of variance uniquely accounted for by each predictor, thus providing an index of relative importance.
Table 6 Summary of linear regression analysis for variables predicting depression in pediatric TLE

BRI=Behavioral Regulation Index; TLE=temporal lobe epilepsy; WCST=Wisconsin Card Sorting Perseverative Errors.
* p<0.05.
Using the BRIEF BRI variable, WCST Perseverative Score, and family stress variables to predict depression, the regression model was significant (R 2=.326; adj R 2=0.291; F3,61=9.35; p<.0001). When common variance among the three predictors was considered, the BRI (partial r=0.282; p=.029) and WCST Perseverative Score (partial r=−0.263; p=.042) both emerged as unique predictors of parent rated depression
Comparing the Impact of Hippocampal Sclerosis on Depression and Executive Funtion
Because EF variables were found to significantly predict parent depression ratings, differences between youth with (n=17) and without HS (n=45) were examined on depression ratings, WCST scores, and the BRIEF BRI. Despite uneven group sample sizes, Levene’s Test revealed the homogeneity of variance assumption was met (p>.10). For youth with a positive identification of HS, depression ratings were in the At-risk range as a group (M=69.94; SD=14.79). WCST performance was in the low average range (M=89.82; SD=17.62), and parent ratings of behavioral regulation on the BRIEF were in the normal range (M=59.88; SD=12.87).
Those youth without HS had depression ratings in the normal range for their age (M=58.20; SD=13.71), WCST Perseverative Errors Score in the average range (M=99.20; SD=16.04), and behavioral regulation ratings at the upper limit of the normal range (62.71; SD=13.31). Because no group differences were identified for seizure-specific variables, sociodemographic characteristics, or intellectual level (p>.05), covariates were not used. ANOVA identified significant group differences for depression ratings (F 1,61=6.63; p=.012) and WCST performance (F 1,61=4.00; p=.048); however, mean differences were not found for the BRI variable (F 1,61=0.57; p=.454).
Exploring WCST as a Mediator Between Hippocampal Sclerosis and Depression
Building on findings from the previous analysis, the degree to which WCST performance mediated the relationship between HS and depression was examined. The presence of HS was significantly associated with greater perseverative errors committed on the WCST (β=-0.25; p=.048), which successfully predicted parental ratings of youth depression (β=-0.41; p=< .001). The previously significant relationship between children with HS and depression (β=0.32; p=.012) was completely attenuated when WCST was included in the model (β=0.22; p=.068), thus fulfilling the requirements of Baron and Kenny’s traditional causal steps approach for statistical mediation (Baron and Kenny, Reference Baron and Kenny1986). The significance of the mediation model was further confirmed by the 95% confidence interval range (95% confidence interval [CI] [0.12, 15.81]) derived by Preacher and Hayes’ bootstrapping procedure for detecting indirect effects (Preacher & Hayes, Reference Preacher and Hayes2004). The results of these analyses are depicted in Figure 2.
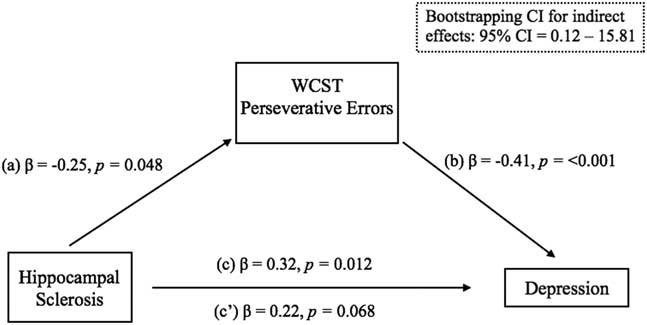
Fig. 2 Multiple linear regression analyses for statistical mediation.
Examining the Impact of HS on Youth Depression
To quantify the extent to which HS served as a specific risk factor for clinically elevated depression ratings, a logistic regression was conducted by dividing the sample according to those with or without HS. The presence of HS was associated with a four-fold increased risk of clinically elevated depression ratings on the BASC-2 (odds ratio=4.42; p=.014; 95% CI for odds ratio [1.36, 14.39]).
DISCUSSION
Children and adolescents with TLE have been shown to be more susceptible to depressive symptoms than youth with other types of epilepsy (Salpekar et al., Reference Salpekar, Berl, Havens, Cushner‐Weinstein, Conry, Pearl and Gaillard2013; Schraegle & Titus, Reference Schraegle and Titus2017a; Titus et al., Reference Titus, Kanive, Sanders and Blackburn2008). Given the deleterious impact of pediatric depressive symptoms on HRQOL (Clary, Vander Wal, & Titus, Reference Clary, Vander Wal and Titus2010; Plioplys, Reference Plioplys2003; Schraegle & Titus, Reference Schraegle and Titus2017a), the present study examined the unique contribution of sociodemographic, epilepsy specific, and neurocognitive factors on depressive features in children and adolescents with TLE. It is hoped that a more complete understanding of these factors will assist with early detection and screening for depression among children and adolescents with TLE.
With regard to the first research question, seizure-specific and sociodemographic factors were only marginally correlated with youth depressive features. For example, aside from a moderate association between HS and depressive symptoms, all other seizure-specific variables were only mildly associated with depressive symptoms. Despite research showing the adverse effect of seizure variables on HRQOL (Devinsky et al., Reference Devinsky, Westbrook, Cramer, Glassman, Perrine and Camfield1999; Schraegle & Titus, Reference Schraegle and Titus2016), these factors were not strongly related to depressive features in our sample. Like seizure factors, most sociodemographic factors, such as maternal education, parental relationship status, and parental psychiatric history, were also marginally related to depressive symptoms. The exception to this was family stress, which was consistent with previous research (Dunn, Austin, & Huster, Reference Dunn, Austin and Huster1999; Oostrom, Schouten, Kruitwagen, Peters & Jennekens-Schinkel, Reference Oostrom, Schouten, Kruitwagen, Peters and Jennekens-Schinkel2001; Pianta & Lothman, Reference Pianta and Lothman1994). This highlights the important role of family stress on child adjustment in pediatric epilepsy.
The current study also demonstrated a significant relationship between cognitive functioning and depression features in youth with TLE. While this relationship was not observed across language, visuospatial reasoning, memory (verbal/nonverbal), and attention domains, a significant relationship was specifically identified in the domain of executive functioning. That is, deficits in behavioral regulation (i.e., BRI) and cognitive flexibility (i.e., WCST perseverative errors) were significantly associated with higher depression ratings. This is a novel finding in a pediatric epilepsy population; however, research conducted with adult TLE samples has demonstrated the significant relationship between WCST perseverative responses and severity of self-reported depressive features (Seidenberg, Hermann, Noe, & Wyler, Reference Seidenberg, Hermann, Noe and Wyler1995).
Espinosa et al. (Reference Espinosa, Machado, González, González, Montoto and Sotomayor2010) reported that greater levels of perseverative errors on the WCST were predictive of suicidality in adults with TLE. These findings suggest that depression symptoms may be related to underlying cognitive processes that regulate emotional and behavioral responses, indicating that youth with epilepsy who are experiencing greater executive dysfunction related to regulatory control may be at higher risk for depression. Moreover, by combining significant EF variables and family stress, it was further discovered that depression ratings were uniquely predicted both by the BRIEF BRI and the WCST perseverative errors variable. While not emerging as a significant predictor, a mild-to-moderate partial correlation was identified for family stress, suggesting an important but less prominent role for this variable.
While the mechanism of action between EF deficits and depression in pediatric TLE is unknown, prospective studies indicate that EF deficits play an active role in precipitating an initial depressive episode in adolescents (Letkiewicz et al., Reference Letkiewicz, Miller, Crocker, Warren, Infantolino, Mimnaugh and Heller2014). In non-clinical samples, trait negative affect has been associated with decreased activity in brain regions involved in top–down control of attention during emotional distraction and with disrupted top–down control of attention on a non-emotional task (Crocker et al., Reference Crocker, Heller, Spielberg, Warren, Bredemeier, Sutton and Miller2012). In this regard, EF deficits contribute to difficulties switching or shifting attention away from affective information, potentially leading to a prolonged focus on negative material. This, in turn, can maintain or increase negative affect and subsequently maintain or increase depression symptoms.
Joormann and Gotlib (Reference Joormann and Gotlib2008) showed that prolonged focus on negative material could be associated with inhibition deficits, which can allow negative material to preoccupy working memory. EF deficits in cognitive flexibility may further serve to alter appraisal processes that may influence both physiological reactivity and subjective distress in response to adverse events. This can play a critical role in youth with epilepsy who, as a group, report higher levels of subjective distress (Chong et al., Reference Chong, Jamieson, Gill, Singh-Grewal, Craig, Ju and Tong2016). In the current sample, emotional control was found to be a significant predictor of depressive features in pediatric TLE. This may reflect an inability to modulate negative thoughts or fears related to epilepsy. When this modulation is poorly controlled, individuals with epilepsy may become overly engaged in emotional reactions and withdraw from goal directed behavior. This process would be compounded by inefficiencies in top–down cognitive control, such as the perseverative tendencies captured by the WCST.
The influence of family stress on depressive features in the present sample is not surprising when considering the elevated coping demands in this population. It is reasonable to expect that youth with epilepsy rely heavily on external resources, such as family, to manage the emotions that accompany their condition. However, when family stress is higher, family members, such as parents, may be less available or may even exacerbate maladaptive youth coping (Schraegle & Titus, Reference Schraegle and Titus2017b). It will be important for future research to investigate the extent to which family stress and other familial factors contribute to youth behavioral regulation in the context of depression in pediatric TLE. It could be that youth emotional control is partially predicted by parental adjustment, which may manifest in epilepsy shaming (Chong et al., Reference Chong, Jamieson, Gill, Singh-Grewal, Craig, Ju and Tong2016) and/or parental helplessness (McLaughlin, Schraegle, Nussbaum, & Titus, Reference McLaughlin, Schraegle, Nussbaum and Titus2016). Thus, adaptive coping in the face of epilepsy with depression is likely to be impacted by several factors. Determining these factors will be important for family based interventions tailored to treat depression among children and adolescents with TLE.
Similar to what has been previously found in the adult and pediatric TLE literature (Hermann & Seidenberg, Reference Hermann and Seidenberg1995; Rzezak et al., Reference Rzezak, Valente and Duchowny2014; Sretton & Thompson, Reference Stretton and Thompson2012), the current study confirmed HS as a risk factor for EF deficits in youth with epilepsy. Specifically, youth with HS were found to have significantly more perseveration errors on the WCST than those without HS. In a sample of adults with TLE, Hermann and Seidenberg (Reference Hermann and Seidenberg1995) demonstrated that abnormal interictal discharge propagation on EEG from mesial temporal structures to the prefrontal cortex was associated with deficits observed on the WCST.
Analogous differences were not observed for parent rated behavioral regulation on the BRIEF. The presence of HS incurred a four-fold increase in the likelihood of clinically significant depressive features in our sample. Although this finding is often observed in the adult TLE literature (Quiske, Helmstaedter, Lux, & Elger, Reference Quiske, Helmstaedter, Lux and Elger2000), to our knowledge, this work marks one of the first demonstrations of this relationship in a pediatric epilepsy sample. Of interest, the relationship between HS and depression was fully mediated by the WCST perseverative errors variable. This finding is important because it offers the first step toward developing a causal mechanism that comprehensively examines HS, EF, and emotional maladjustment. Longitudinal research is necessary to verify the directionality of these relationships.
Recently, Kemmotsu et al. (Reference Kemmotsu, Kucukboyaci, Cheng, Girard, Tecoma, Iragui and McDonald2013) demonstrated that depressive features in adult TLE was significantly associated with frontotemporal network dysfunction indicated by altered functional connectivity between the left hippocampus and left anterior prefrontal cortex (Brodmann area 10). Corcoran and Upton (Reference Corcoran and Upton1993) postulated that HS alone could explain executive dysfunction by compromising its role as a comparator of actions, wherein information about a previous response is used to guide future behavior via working memory.
Conversely, Hermann, Wyler, and Richey (Reference Hermann, Wyler and Richey1988) attributed EF deficits in TLE to frontal lobe dysfunction caused by propagation of “neural noise” from the temporal lobe, especially in the case of a mesial temporal seizure focus through pathways that connect the anterior temporal lobe and hippocampus with frontal regions. These two theories are now considered complimentary given that both short and long-range networks are altered by limbic system pathology. Of interest, similar morphological changes to the hippocampus, amygdala, and frontal networks are often observed in adults with MDD (Keller et al., Reference Keller, Baker, Downes and Roberts2009; Sheline, Gado, & Kraemer, Reference Sheline, Gado and Kraemer2003). For this reason, depression and TLE has been described as an epiphenomenon (Valente & Busatto Filho, Reference Valente and Busatto Filho2013).
The significant and unique role of executive dysfunction in depression among youth with TLE elucidates another potential avenue for therapeutic intervention. Because sociodemographic and seizure-specific factors are not always modifiable, the potential of modifying depression risk or severity by targeting cognition, and specifically executive functioning, is intriguing. The presence of poor behavioral regulation as well as impaired cognitive flexibility and problem solving may be important in the development and maintenance of depression in this population.
The current work suggests a role for EF screening early in the condition to initiate intervention and/or minimize the potential for internalizing psychopathology. Because deficits in EF have also been found to be significant barriers for HRQOL in pediatric epilepsy (Schraegle & Titus, Reference Schraegle and Titus2016), attempting to modify these abilities may represent an area through which intervention could have a meaningful impact on functional outcomes. The types of executive dysfunction exhibited in the current study, namely emotional control and cognitive flexibility, provide insights into how cognitive-based interventions may improve coping and ultimately HRQOL in youth with TLE.
Some limitations to the present study should also be considered. All children and adolescents were referred for epilepsy treatment at a tertiary care center, which may reflect a greater severity of epilepsy. As such, these findings may be especially useful for those in similar settings, but may not generalize to those children and adolescents with less severe forms of TLE. The present study relied upon parent-report of depression features in children and adolescents with depression. This was done to avoid exclusion of patients of younger age, lower functioning, and higher epilepsy severity.
However, sole reliance on parent-report may have resulted in bias that should be clarified through future research. Moreover, family stress was examined by a single Likert item and dichotomized in our first analysis, which may have constricted sample variability. Future studies using more comprehensive rating measures of family stress are encouraged in future work on this topic. Lastly, morphological changes in the hippocampus were defined by the presence or absence of HS. Volumetric analyses were not conducted and, as such, the relationship of hippocampal volume with depression in pediatric TLE was not investigated.
The current study highlights and helps to define the strong association between executive functioning and parent rated depression in a sample of children and adolescents with TLE. While HS was found to be a predisposing factor for depression symptoms, EFs related to cognitive flexibility were found to mediate this relationship. Regular screening for executive dysfunction may help identify risk factors for depression to allow for prevention or early intervention. The current findings also suggest a potential mechanism for improving HRQOL outcomes and minimizing the risk for depression in youth with epilepsy by working to remediate EF deficits (e.g., Crocker et al., Reference Crocker, Heller, Warren, O’Hare, Infantolino and Miller2013). Intervention strategies aimed at improving emotional control and cognitive flexibility may improve effective coping strategies, which may in turn contribute to the prevention of depression and ultimately improve the lives of children and adolescents with TLE.
ACKNOWLEDGEMENTS
None of the authors received funding for this project. None of the authors have any conflicts of interest to disclose. We confirm that we have read the journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.










