INTRODUCTION
A substantial body of literature suggests that individuals with eating disorders (ED), such as anorexia nervosa (AN) and bulimia nervosa (BN), are dissatisfied with their physical appearance (Bruch, Reference Bruch1962; Farrell, Lee, & Shafran, Reference Farrell, Lee and Shafran2005; Phillipou et al., Reference Phillipou, Rossell, Gurvich, Castle, Troje and Abel2016). Most of these studies rely on explicit self-report measures. While such measures are useful, they are subject to well-known drawbacks, including susceptibility to socially desirable answering (Dovidio, Kawakami, & Gaertner, Reference Dovidio, Kawakami and Gaertner2002; Gawronski, LeBel, & Peters, Reference Gawronski, LeBel and Peters2007) and participants’ limited access to their own underlying cognitive processes (Gawronski et al., Reference Gawronski, LeBel and Peters2007). Implicit tasks, in which one’s beliefs and attitudes are inferred rather than directly interrogated, represent an alternative approach. Although the exact cognitive processes underlying implicit tasks are not fully known (Gawronski et al., Reference Gawronski, LeBel and Peters2007), they have been shown to provide predictive value above and beyond traditional self-report measures in a range of disciplines (Asendorpf, Banse, & Mücke, Reference Asendorpf, Banse and Mücke2002; Hugenberg & Bodenhausen, Reference Hugenberg and Bodenhausen2004; Neumann, Hülsenbeck, & Seibt, Reference Neumann, Hülsenbeck and Seibt2004; Vartanian, Polivy, & Herman, Reference Vartanian, Polivy and Herman2004).
Explicit techniques have been widely used in the eating disorder literature, however, research conducted in the field of body representations suggests that the internal representation of the body (i.e., the body schema) is most typically a pre-conscious entity (Dijkerman & de Haan, Reference Dijkerman and de Haan2007; Schwoebel & Coslett, Reference Schwoebel and Coslett2005). This aspect of the body schema complicates its assessment using explicit measures. Commonly used explicit techniques include both video (Smeets, Ingleby, Hoek, & Panhuysen, Reference Smeets, Ingleby, Hoek and Panhuysen1999) and picture distortion methods (Hagman et al., Reference Hagman, Gardner, Brown, Gralla, Fier and Frank2015) in which video or photographic images of the participant are digitally altered. Participants are then asked to judge whether the distorted images are smaller, larger, or the same size as themselves, that requires participants to compare their internal body schema with an external visual stimulus. Such a task may yield information regarding the comparison between the internal representation and the stimulus, but may not yield direct information regarding the internal representation. Thus, an implicit task, such as the one described here, may be more appropriate in directly probing of the body schema.
While an explicit task alone may not be suited for interrogation of a pre-conscious construct, there is evidence that implicit and explicit tasks together may provide complementary information in participants with ED (Cockerham, Stopa, Bell, & Gregg, Reference Cockerham, Stopa, Bell and Gregg2009; Cserjési et al., Reference Cserjési, Vermeulen, Luminet, Marechal, Nef, Simon and Lénárd2010). For example, Phillipou et al. (Reference Phillipou, Rossell, Gurvich, Castle, Troje and Abel2016) used an implicit task in which participants were asked to identify the affect of a point-light biological motion figure. These “figures” are simply patterns of moving dots representing a human body in motion and can be adjusted on a range of variables, including gender, weight, and affect (Phillipou et al., Reference Phillipou, Rossell, Gurvich, Castle, Troje and Abel2016). Participants with ED were significantly worse at identifying “sad” affect in the figures, compared to HC participants (Phillipou et al., Reference Phillipou, Rossell, Gurvich, Castle, Troje and Abel2016). Zucker et al. (Reference Zucker, Moskovich, Bulik, Merwin, Gaddis, Losh and LaBar2013) used an implicit task to investigate the relationship between recovery status and affect perception, again using point-light figures. Participants were either currently diagnosed with AN or had been previously diagnosed, but were weight-recovered and no longer met diagnostic criteria. Participants with a current AN diagnosis were significantly worse at identifying the affect of point-light figures compared to weight-recovered individuals (Zucker et al., Reference Zucker, Moskovich, Bulik, Merwin, Gaddis, Losh and LaBar2013), indicating that disruption of affect perception may be ameliorated with weight-recovery. Although these point-light figures differ greatly from the current MMI task, these studies provide clear evidence that implicit tasks yield valuable, and often complementary, information in the study of eating disorders.
We report an investigation of participants’ body schemas using an implicit task. More specifically, we asked healthy control subjects (HC) and participants with ED to perform a task in which completed actions were compared to mental simulations of the same action. Just as one can execute an action, one can simulate the same action; that is, one is able to imagine touching one’s ear without actually moving at all. Converging lines of evidence from behavioral (Jeannerod, Reference Jeannerod1995, Reference Jeannerod2001, Parsons, Reference Parsons1987, Reference Parsons1994), functional neuroimaging (Grafton, Arbib, Fadiga, & Rizzolatti, Reference Grafton, Arbib, Fadiga and Rizzolatti1996; Grèzes & Decety, Reference Grèzes and Decety2001), and transcranial magnetic stimulation studies (Ganis, Keenan, Kosslyn, & Pascual-Leone, Reference Ganis, Keenan, Kosslyn and Pascual-Leone2000; Rossini, Rossi, Pasqualetti, & Tecchio, Reference Rossini, Rossi, Pasqualetti and Tecchio1999) all suggest that covert actions are physiologically similar to completed actions, short of being physically enacted by the motor system.
Thus, when participants are asked to imagine performing an action, we can be reasonably certain that they are, in fact, mentally simulating that action. Therefore, if individuals with ED have a distortion of the body schema such that they imagine portions of their body to be larger than they truly are, they will take longer to imagine tracing the enlarged body parts because they will be mentally tracing a greater distance along or around the enlarged body, as compared to the actual body part.
Work exploring the use of mental motor imagery (MMI) tasks in patient populations suggests that MMI functions similarly in these groups and healthy individuals (Coslett, Reference Coslett1998; Schwoebel & Coslett, Reference Schwoebel and Coslett2005). Given that the current study represents the first use of an MMI task in a psychiatric population, below is a description of a similar MMI task used in a population with chronic pain. Coslett and colleagues asked participants with chronic leg (Coslett, Medina, Kliot, & Burkey, Reference Coslett, Medina, Kliot and Burkey2010a) or arm (Coslett, Medina, Kliot, & Burkey, Reference Coslett, Medina, Kliot and Burkey2010b) pain to judge the laterality of a visually depicted right or left foot or hand. Consistent with the observation that pain is associated with slowing of movements, they found that participants with chronic limb pain were significantly slower at judging the laterality of the painful hand (Coslett et al., Reference Coslett, Medina, Kliot and Burkey2010b) or foot (Coslett et al., Reference Coslett, Medina, Kliot and Burkey2010a) despite the task requiring no movement. Reflecting the fact that movement of a painful limb often exacerbates the pain, participants with chronic limb pain are slower at judging the laterality of the extremity. Of particular note, the observed decrement in judgment time can be improved following treatment which decreases pain in the affected body part (Schwoebel, Coslett, Bradt, Friedman, & Dileo, Reference Schwoebel, Coslett, Bradt, Friedman and Dileo2002).
Motivated by the limitations of self-report (Dovidio et al., Reference Dovidio, Kawakami and Gaertner2002; Gawronski et al., Reference Gawronski, LeBel and Peters2007) and by the promise of implicit MMI tasks, we sought to determine if MMI could be used with participants with ED to assess the real-time, often pre-conscious (Coslett, Saffran, & Schwoebel, Reference Coslett, Saffran and Schwoebel2002; Schwoebel, Boronat, & Coslett, Reference Schwoebel, Boronat and Coslett2002; Schwoebel & Coslett, Reference Schwoebel and Coslett2005) body representation, hereafter the “body schema”. We reasoned that if MMI indexes the body schema and if this representation is systematically distorted in individuals with ED, one would expect their performance on MMI tasks to reflect this distortion.
For example, as people with AN often envision their stomachs to be more protruding than they actually are (Smeets & Kosslyn, Reference Smeets and Kosslyn2001), one would expect the time taken to imagine moving their finger across their abdomen to be longer than the time taken to actually perform the same movement. Furthermore, aberrant performance on MMI tasks would be expected to be selective to body parts such as the abdomen, which are most likely to be distorted for individuals with ED.
In contrast, individuals with ED would not be expected to distort the circumference of their heads. Thus, we expected participants with ED, but not HC participants, to take longer to imagine tracing body parts that are likely to be sensitive to the distortions of an eating disorder, compared to body parts that are not likely to be sensitive to those distortions. Furthermore, we sought to determine how our MMI task correlated with more traditional self-report measures of body satisfaction and whether our MMI task provided information above beyond those traditional measures. To our knowledge, this work represents the first effort to use an implicit MMI task in the assessment of differences in real-time representations of the body schema. Of importance, this MMI task could provide important supplementary clinical information regarding the underlying body schema distortion in individuals with ED.
Previous work in the eating disorder literature has been plagued by a lack of consensus in the language used to describe and investigate body distortions (Banfield & McCabe, Reference Banfield and McCabe2002; Cash & Deagle, Reference Cash and Deagle1997). We use the term body schema here to refer to the real-time, typically pre-conscious representation of one’s body in space. According to Head and Holmes’ (Reference Head and Holmes1911) seminal conceptualization of the construct, the body schema is a mental representation of the body in space, incorporating information about the positions of body parts in relation to each other and to the world around us. This representation is updated in real-time with information from a variety of sensory modalities (e.g., vision, proprioception, audition, tactile, etc.). In contrast, “body image” encompasses both a “perceptual” and an “affective/cognitive” aspect (Cornelissen, Johns, & Tovée, Reference Cornelissen, Johns and Tovée2013; Schwoebel & Coslett, Reference Schwoebel and Coslett2005) and includes feelings and attitudes about one’s body. In contrast, the body schema is not associated with feelings or judgements about one’s body.
METHODS
Participants
A total of 82 women participated. Forty-two were individuals with ED who were recruited from a residential ED treatment center and ranged in age from 18–63 years (M=28.6 years; SD=11.4 years). Additionally, 40 healthy women participated, ranging in age from 18–61 years (M=25.21 years; SD=10.73 years). Healthy control participants were recruited from the University of Pennsylvania campus and surrounding by posting flyers advertising the study. A t test revealed the groups did not differ in age (p=.17). Due to the extensive clearance process to gain access to the residential treatment facility, data collection occurred between 2013 and 2015.
Using DSM-IV-TR criteria (American Psychiatric Association, 2000), a psychiatrist with expertise in ED assigned each participant a provisional diagnosis at the time of their admission to the center. Individuals were assigned a final diagnosis at the time of discharge. All diagnoses were assigned by a trained psychiatrist on staff at the center and were based on the Eating Disorder Examination (Fairburn & Cooper, Reference Fairburn and Cooper1993). The 42 participants with ED included 20 individuals with AN, 13 with BN, 9 with eating disorder not otherwise specified (EDNOS), and 1 with binge eating disorder (BED). The BED participant was excluded from further analyses because her body mass index (BMI) was a statistical outlier in our sample, including HC participants. Following removal of this one participant, BMIs for all participants (for both Healthy Control participants and participants with ED) ranged from 16.51–29.83 (M HC=22.17; SD HC=4.43; M ED=20.86; SD ED=6.60).
Procedure
All procedures were conducted with the approval of the Institutional Review Board of the University of Pennsylvania and in accordance with the Declaration of Helsinki. During recruitment, participants were informed that the study sought to investigate how people make movements and how people imagine making movements. Upon arrival at the study session participants provided informed consent, then completed a series of questionnaires pertaining to body satisfaction, including the Body Shape Questionnaire (BSQ; Cooper, Taylor, Cooper, & Fairbum, 1987). After filling out the questionnaires, all participants completed the MMI tasks, described in more detail below. Healthy participants’ height and weight were recorded at the end of the session, following the completion of the questionnaires and the MMI task. For individuals with ED, height and most recent weight were obtained from their chart information. The entire session lasted approximately 30 minutes.
MMI Tasks
In different blocks of trials, participants were asked to execute a movement or to imagine executing the same movement; three movements involved sensitive body parts (abdomen, buttocks, thigh), three involved neutral body parts (shin, forearm, head), and one involved a non-body object (wood block), for a total of seven movements. The three sensitive body parts were chosen specifically because they are thought to be sensitive to the distortions of an eating disorder, while the neutral body parts were chosen because they were thought to be less sensitive to such distortions. For example, participants with eating disorders do not often state that their heads are too large, whereas they may be concerned about the size of their abdomen. Evidence from the eating disorder literature suggests that participants with ED do not differ from control participants in their perception of non-body objects, such as a block, a vase, or a mannequin (Farrell et al., Reference Farrell, Lee and Shafran2005; Whitehouse, Freeman, & Annandale, 1988), and there is no reason to suspect that individuals with eating disorders should have altered mental imagery in general. However, the non-body control object was included to address that possibility.
All seven movements were completed in two conditions using an ABBA block design: in condition A participants actually performed the movements, in condition B participants imagined making the same movements. This order was chosen so participants had experience making the movements before being asked to imagine them. The order of body parts was randomized within each block for each participant and the researcher demonstrated each movement to the participant the first time the subject was asked to execute it. There were two trials per body part in each block, which were averaged together, yielding a total of four real and four imagined trials per body part per participant. Tracings were always completed with the right hand and with eyes closed. We note that in previous work with normal subjects and participants with chronic pain, there was no difference in performance as a function of handedness (Coslett et al., Reference Coslett, Medina, Kliot and Burkey2010b, Reference Coslett, Medina, Kliot and Burkey2010a). In all conditions, real or imagined, the researcher said “start” to begin the trial and the participant said “stop” upon completion of the tracing. All MMI procedures were identical for both HC participants and participants with ED. Below is a description of each body part tracing:
Abdomen
While standing, participants started with the tip of the index finger on one hip, traced along the surface of the abdomen to the other hip, and then traced back to the starting point. For this and all other tasks, participants performed the movement (or imagined movement) three times on each trial.
Buttocks
While standing, participants started with the tip of the index finger on the left posterior iliac crest, with the forearm behind the body, traced across the buttocks to the right iliac crest, and then traced back to the starting point.
Thigh
While seated, participants started with the tip of the right index finger on the left thigh, approximately 15 cm above the knee. Participants traced in one direction around the circumference of the thigh until they returned to the starting position, then traced around the circumference of the thigh in the opposite direction, finally ending at the initial starting position.
Shin
While seated with feet flat on the ground, participants started with the right index finger below the kneecap. When instructed, participants traced along the surface of the shin, down to the top of the foot, and back to the starting position.
Forearm
While sitting or standing, participants started with the left forearm raised vertically in front of them, with palm facing inward (so the dorsal aspect of the forearm was facing outward). Participants traced along the dorsal aspect of the forearm from one end to the other, concluding at the initial starting position.
Head
While sitting or standing, participants began with the index finger in the middle of the forehead, traced along the surface of the skull in a clockwise direction (as viewed from above) until reaching the starting point, then traced in a counterclockwise direction until reaching the starting point again.
Block
Participants began with the index finger on one end of the block, traced to the other end of the block, then traced back to the starting point.
As described previously, the abdomen, buttocks, and thigh were hypothesized to be sensitive to the distortions of an ED (sensitive), while the shin, forearm, and head were thought to be insensitive to these distortions (control).
Statistical Analysis
Data from the two real and two imagined blocks were averaged to create one real and one imagined tracing time per participant for each of the seven conditions (six body parts and 1 block). A ratio of imagined to real tracing time was calculated for each condition, with values over 1 indicating a longer tracing time for imagined than for real movements. This was done to control for individual differences in raw times required to imagine or execute the movements. Most analyses were conducted by collapsing across the movements into three conditions: “control,” “sensitive,” and “block.” Analyses where the data are grouped differently are clearly delineated below.
First, a paired t test examined whether ratios in the control condition (head, shin, and forearm) differed from ratios in the block condition (non-body) for all participants. For this analysis, the control conditions and block condition were compared across the entire group of participants. A 2 × 2 repeated measures analysis of variance (ANOVA) was conducted with diagnosis (HC vs. ED) as the between-subjects factor and condition (sensitive vs. control) as the within-subjects factor. The ratio of real:imagined tracing served as the outcome. Given our hypothesis, we predicted an interaction between diagnosis and condition. T tests were conducted as post hoc tests to determine whether ratios for control and sensitive body parts differed within the HC and ED groups, as this is not directly examined by an ANOVA. Post hoc t tests were corrected using a Bonferroni correction (0.05/4 tests=0.0125 as the new critical p-value for post hoc t tests). Correlations between ratios (sensitive/control and sensitive alone) and BSQ score were conducted to determine how our novel MMI task related to a more traditional self-report assessment.
Finally, three separate logistic regressions were performed to determine: (a) if diagnosis could be predicted by MMI alone (ratio of sensitive to control), (b) if diagnosis could be predicted by MMI (ratio of sensitive to control) above and beyond the BSQ, and (c) if diagnosis could be predicted by MMI (sensitive body parts alone) above and beyond the BSQ. Finally, the three models were compared to determine which model yielded the best fit and explained the maximal variance. All statistical analyses were performed using R (version 3.2.2).
RESULTS
Ratios were normally distributed, both when collapsed across group, and when split into HC and ED groups (p>.05 for all Shapiro Wilk statistics). A paired t test contrasting ratios for all participants in the control and block conditions revealed no significant difference between ratios (t(79)=1.41; p=.16; d=0.16), suggesting that MMI of body-centered neutral items functions similarly to MMI of non-body neutral items for all participants. As the current study is concerned with the comparison of body-centered MMI, block ratios were not included in subsequent analyses. Additionally, ratios of sensitive to control body parts across all participants were not correlated with BMI in our sample (r(81)=0.12; p=.264); therefore, BMI was also not included in subsequent analyses.
Means and SDs for HC and ED participants can be found in Table 1. The ANOVA revealed a main effect of diagnosis (F(1,162)=12.54; p<.001; partial η 2 =0.10) such that, collapsed across condition, participants with ED have significantly higher ratios of imagined:real movements (M=1.10; SD=0.16) compared to HC participants (M=0.99; SD=0.11). The main effect of condition was not significant (p>.05) and the interaction was not significant (p>.05). Post hoc t tests revealed no significant difference in ratios for the control and sensitive conditions (t(40)=1.23; p=.23; d=0.19) for HC participants.
Table 1 Means and standard deviations for ratios of imagined to real tracing of body parts

Note: N for ED group is 42, N for HC group is 40, and total N is 82.
Participants with ED, in contrast, did show a significant difference in ratios for control and sensitive conditions (t(41)=4.29; p<.0125; d=0.66), such that they took longer to imagine tracing sensitive compared to control body parts.Footnote 1 An independent samples t test revealed that ED participants took longer to imagine tracing sensitive body parts (M=1.24) compared to HC participants (M=1.02), t(81)=3.42, p<.0125, d=0.75). Visual inspection indicated that ED participants also took slightly longer in the control condition (M=1.12) than did their HC counterparts (M=1.04). Although a post hoc t test revealed the difference was not significant (t(81)=1.47; p=.15; d=0.32; see Figure 1), the ratio of sensitive to control body parts is conflated with a slightly higher ratio for control body parts for participants with ED.
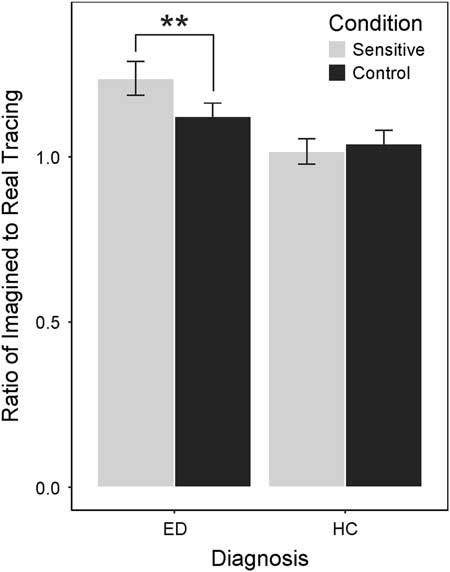
Fig. 1 Histogram of mean ratios for each condition within ED and HC groups. The difference between sensitive and control ratios is significant only for the ED group, the difference is not significant for the HC group. **p<.01
Furthermore, as discussed in the introduction, participants with ED were not expected to differ from HC participants on tracings of control body parts. To obtain a more complete understanding of the results, we used both the ratio of sensitive to control and the ratios for sensitive body parts alone (ratio of Imagined to Real movements for sensitive body parts) as predictor variables for the following analyses. There was a moderate significant correlation between scores on the BSQ and the MMI ratio of sensitive to control body parts, r(81)=0.39, p<.01. This correlation was still significant, although slightly weaker, when only the sensitive body parts were considered (thus omitting the effect of the control body parts), r(81)=0.25, p<.01.
The results of the binomial logistic regression examining the predictive power of ratios of sensitive to control body parts on differentiation between ED and HC participants were statistically significant and provide a 22% proportional reduction in error, b=6.91, SEb=2.08, Z=3.33, p<.01, Nagelkerke R2=0.22. Thus, for every 1-unit increase in the ratio of sensitive to control, the log odds of having an ED diagnosis increase by 6.91.
To determine if MMI contributes unique variance to the discrimination between ED and HC participants above and beyond BSQ scores, two additional logistic regressions were performed. When using the ratio of sensitive to control, MMI contributed marginal variance to the prediction of an ED, b=4.64, SEb=0.27, Wald Z=1.71, p=.087, while the BSQ explained a significant proportion, b=0.05, SE=0.01, Wald Z=4.65, p<.01 (Nagelkerke R2=0.73). However, when only the ratios of sensitive body parts (i.e., abdomen, buttocks, and thigh) were included, MMI significantly contributed unique variance above and beyond the BSQ, b=3.18, SEb=1.51, Wald Z=2.10, p=.035 (Nagelkerke R2=0.75). Model comparisons using likelihood ratios, as well as direct comparisons of pseudo-R2 values, suggest that model 3, which includes the sensitive ratio and BSQ scores, is the most effective model, p<.001, providing a 75% proportional reduction error.
DISCUSSION
Results from the current study support our prediction that participants with ED, but not HC participants, take significantly longer to imagine tracing sensitive body parts compared to control body parts, suggesting that participants with ED implicitly believe those parts to be larger than they really are. Our results also support the idea that MMI tasks contribute information above and beyond traditional self-report measures, such as the BSQ.
The finding of a distortion in the body schema of individuals with ED is in line with the general consensus in the literature (Bruch, Reference Bruch1962; Gleaves & Eberenz, Reference Gleaves and Eberenz1993; Williamson, White, York-Crowe, & Stewart, Reference Williamson, White, York-Crowe and Stewart2004). Here, however, we have used a novel implicit task to more directly assess the body schema. We found a significant moderate-sized correlation between ratios of sensitive to control body parts and scores on the BSQ, which is consistent with evidence suggesting that implicit and explicit tasks interrogate complementary, although separate, constructs (Vartanian et al., Reference Vartanian, Polivy and Herman2004). Additionally, we found that ratios from the MMI task contributed unique variance above and beyond the BSQ, again in line with the literature suggesting that implicit tasks provide supplementary information not tapped by explicit measures (Vartanian et al., Reference Vartanian, Polivy and Herman2004). This finding also argues for the utility of implicit measures in the assessment of individuals’ body schema distortions.
Previous work using MMI tasks suggest that they can be used to aid in the identification of psychological factors such as denial or malingering in pain populations (Coslett et al., Reference Coslett, Medina, Kliot and Burkey2010b; Maruff & Velakoulis, Reference Maruff and Velakoulis2000). A central concern in the treatment of ED is resistance to treatment and the ego-syntonic nature of the disorder (Vitousek, Watson, & Wilson, Reference Vitousek, Watson and Wilson1998; Zucker et al., Reference Zucker, Moskovich, Bulik, Merwin, Gaddis, Losh and LaBar2013). Individuals with AN, in particular, often deny the existence of an eating-related problem (Vartanian et al., Reference Vartanian, Polivy and Herman2004) and sometimes use perception control as a technique to keep their disorder concealed (Vitousek, Daly, & Heiser, Reference Vitousek, Daly and Heiser1991; Vitousek & Manke, Reference Vitousek and Manke1994). Because of these concerns, an implicit task may be particularly important in accessing beliefs and mental constructs that cannot be interrogated explicitly.
Although the current study does not speak directly to this point, it is possible that our MMI task may be useful as a dynamic measure of the body schema, potentially being used both during the initial identification of individuals with ED and as a measure of response to treatment or change in body schema. It has been well established in the ED literature that increased body size estimation (a concept which parallels the distortion in body schema investigated here) contributes to both the development (Farrell et al., Reference Farrell, Lee and Shafran2005) and the maintenance (Bruch, Reference Bruch1962; Cornelissen et al., Reference Cornelissen, Johns and Tovée2013; Slade, Reference Slade1985) of eating disorders.
Previous work has also found that the severity of this increase is a predictor of poor treatment outcome (Couturier & Lock, Reference Couturier and Lock2006; Vocks, Legenbauer, Rüddel, & Troje, Reference Vocks, Legenbauer, Rüddel and Troje2007) and its persistence following treatment puts individuals at a higher risk of relapse (Cash & Deagle, Reference Cash and Deagle1997; Fairburn, Peveler, Jones, & Hope, 1993). The dynamic aspect of the current task would be important in the identification of people who may need more support following discharge or completion of treatment. We note that there is precedent for the claim that MMI tasks may provide a dynamic measure of the body schema and for using implicit measures to track and gauge recovery (Phillipou et al., Reference Phillipou, Rossell, Gurvich, Castle, Troje and Abel2016; Zucker et al., Reference Zucker, Moskovich, Bulik, Merwin, Gaddis, Losh and LaBar2013). Our group has previously demonstrated that the abnormal pattern of performance on the hand laterality MMI task, described above, improved after treatment which reduced the pain in the affected limb (Schwoebel, Coslett, et al., Reference Schwoebel, Coslett, Bradt, Friedman and Dileo2002). Future work should seek to explore this possible utility of the novel MMI task presented here.
Some studies in the ED literature have found a link between subject BMI and the degree of increase in body size estimation (Cornelissen et al., Reference Cornelissen, Johns and Tovée2013); we did not find this relationship in the current study. However, our measurement technique fundamentally incorporates a participant’s actual body size by comparing imagined to real movement times, thereby incorporating individual differences within the measurement itself and eliminating a potential confound that some have argued does not actually reflect the severity of an ED (Machado, Grilo, & Crosby, Reference Machado, Grilo and Crosby2017).
Limitations and Future Directions
Despite novel and compelling findings, the current study is not without limitations. Our ED population was recruited from a private, residential treatment center where staff psychiatrists assign a provisional diagnosis upon admission and a final diagnosis upon discharge. Because of this procedure, diagnoses are not validated by other psychiatrists and we do not have data on diagnosis reliability. However, given that our sample was drawn from a residential treatment center, there can be little argument that these individuals did, in fact, have a clinically meaningful eating-related disorder. Our hypotheses and analyses were based on the general designation of “eating disordered” or “not eating disordered,” rather than specific subtypes of ED, thus deemphasizing this particular issue. However, future work should investigate whether differences in performance exist on this MMI task between ED subtypes. Additionally, it is possible that our results would not generalize to other populations. Future research should seek to investigate this empirical question.
Additionally, we do not have clinical data regarding ED participants’ behaviors or medication information for our participants. It is possible that further separating groups by diagnosis subtype or by clinical behavior could reveal something significant about differences between those groups. Alternatively, however, there are those who have argued that eating disorders are not discrete conditions encapsulated by a categorical diagnosis but are, in reality, dimensional in nature (Widiger & Samuel, Reference Widiger and Samuel2005). Thus, a continuous measure of eating pathology may reveal a relationship with eating disorder severity and body schema distortion as assessed by this task, above and beyond presence (or absence) of an ED diagnosis. As discussed above, the fact that our population was drawn from a residential treatment center, along with the generalized nature of our hypotheses, together serve to deemphasize the impact of clinical data in this study. Medications would not be expected to alter one’s mental imagery; however, future work should investigate the consequence of clinical data and the effects of medication on this task.
In our recruitment of HC participants, we were required to disclose that we planned on weighing them and measuring their height. This could have inadvertently resulted in a self-selected population skewed toward being quite satisfied with their bodies while selecting out those participants who may not be as comfortable about their bodies. Again, future work replicating this study in different communities and populations would validate the MMI task and provide normative data, further increasing its utility.
Finally, the MMI task used here may be applicable to other conditions characterized by an incongruity between actual and perceived body form. For example, individuals with body dysmorphic disorder with muscle dysmorphia tend to feel their bodies are too small or are not muscular enough (American Psychiatric Association, 2013). As such, their mental representation of particular muscle groups would be expected to be smaller than their actual size. With respect to the current MMI task, they would be expected to take a shorter time to imagine tracing affected body parts. Future work should explore this and other possible applications of the novel, implicit MMI task described here.
CONCLUSIONS
In summary, the current study used a novel, implicit MMI task and found evidence of a body schema distortion for participants with eating disorders, but not for healthy control participants. Furthermore, ratios for sensitive body parts provide additional information above and beyond the BSQ, a traditional self-report measure of body satisfaction. Information gleaned from the current task about underlying body schema distortions may provide additional, valuable clinical information in the treatment of eating disorders.
ACKNOWLEDGMENTS
The authors thank Susan Hao for her assistance in coding and inputting the data. None of the authors have any conflicts of interest to disclose. This work was supported by the William N. Kelley Chair of Neurology (H.B.C.).




